Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells
- PMID: 15100267
- PMCID: PMC2842445
- DOI: 10.4049/jimmunol.172.9.5287
Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells
Abstract
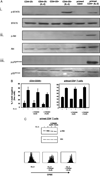
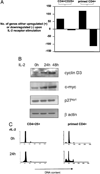
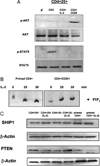
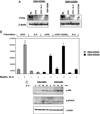
Despite expression of the high-affinity IL-2R, CD4(+)CD25(+) regulatory T cells (Tregs) are hypoproliferative upon IL-2R stimulation in vitro. However the mechanisms by which CD4(+)CD25(+) T cells respond to IL-2 signals are undefined. In this report, we examine the cellular and molecular responses of CD4(+)CD25(+) Tregs to IL-2. IL-2R stimulation results in a G(1) cell cycle arrest, cellular enlargement and increased cellular survival of CD4(+)CD25(+) T cells. We find a distinct pattern of IL-2R signaling in which the Janus kinase/STAT pathway remains intact, whereas IL-2 does not activate downstream targets of phosphatidylinositol 3-kinase. Negative regulation of phosphatidylinositol 3-kinase signaling and IL-2-mediated proliferation of CD4(+)CD25(+) T cells is inversely associated with expression of the phosphatase and tensin homologue deleted on chromosome 10, PTEN.
Figures

References
-
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105. - PubMed
-
- Refaeli Y, Van Parijs L, London CA, Tschopp J, Abbas AK. Biochemical mechanisms of IL-2-regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615. - PubMed
-
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice: implications for the nonredundant function of IL-2. Immunity. 2002;17:167. - PubMed
-
- Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+T cells: IL-2Rα and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J. Immunol. 2002;169:4850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

