Effectiveness of implementing the agency for healthcare research and quality smoking cessation clinical practice guideline: a randomized, controlled trial
- PMID: 15100337
- PMCID: PMC7666639
- DOI: 10.1093/jnci/djh103
Effectiveness of implementing the agency for healthcare research and quality smoking cessation clinical practice guideline: a randomized, controlled trial
Abstract
Background: The Agency for Healthcare Research and Quality (AHRQ) Smoking Cessation Clinical Practice Guideline recommends that all clinicians strongly advise their patients who use tobacco to quit.
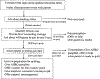
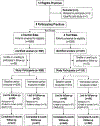
Methods: We conducted a randomized, controlled trial of the effectiveness of Guideline implementation at eight community-based primary care clinics in southern Wisconsin (four test sites, four control sites) among 2163 consecutively enrolled adult patients who smoked at least one cigarette per day and presented for nonemergency care during the baseline period (June 16, 1999, to June 20, 2000) or the intervention period (from June 21, 2000, to May 3, 2001). After collecting baseline data, staff at test sites implemented the intervention over a 2-month period. The intervention included a tutorial for intake clinicians, group and individual performance feedback for intake clinicians, use of a modified vital signs stamp, an offer of free nicotine replacement therapy, and proactive telephone counseling. Staff at control sites received only general information about the AHRQ Guideline. Self-reported abstinence from smoking was determined by telephone interviews at 2- and 6-month follow-up assessments. Hierarchical logistic regression models were used to estimate the odds ratios (ORs) for treatment assignment after adjustment for patient characteristics. All statistical tests were two-sided.
Results: There were no statistically significant differences in smoking cessation rates between participants at test and control sites during the baseline period. Among participants treated during the intervention period, those at test sites were more likely than those at control sites to report being abstinent at the 2-month (16.4% versus 5.8%; adjusted OR = 3.3, 95% confidence interval [CI] = 1.9 to 5.6; P<.001) and 6-month (15.4% versus 9.8%; adjusted OR = 1.7, 95% CI = 1.2 to 2.6; P =.009) follow-up assessments and to report continuous abstinence, that is, abstinence at both 2 and 6 months (10.9% versus 3.8%; adjusted OR = 3.4, 95% CI = 1.8 to 6.3; P<.001).
Conclusion: Implementation of a guideline-based smoking cessation intervention by intake clinicians in primary care is associated with higher abstinence among smokers.
Figures
Comment in
-
Tobacco control in the physician's office: a matter of adequate training and resources.J Natl Cancer Inst. 2004 Apr 21;96(8):573-5. doi: 10.1093/jnci/djh128. J Natl Cancer Inst. 2004. PMID: 15100328 No abstract available.
References
-
- Fiore M, Bailey W, Cohen S, Dorfman S, Goldstein M, Gritz E, et al. Treating tobacco use and dependence Clinical Practice Guideline. Rock-ville (MD): U.S. Department of Health and Human Services, U.S. Public Health Service; 2000. Available at: http://www.surgeongeneral.gov/tobacco/treating_tobacco_use.pdf. [Last accessed: March 6, 2004.]
-
- Physician and other health-care professional counseling of smokers to quit--United States, 1991. MMWR Morb Mortal Wkly Rep 1993;42:854–7. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00022151.htm. [Last accessed: March 9, 2004.] - PubMed
-
- Jaen CR, Stange KC, Tumiel LM, Nutting P. Missed opportunities for prevention: smoking cessation counseling and the competing demands of practice. J Fam Pract 1997;45:348–54. - PubMed
-
- Thorndike AN, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA 1998;279:604–8. - PubMed
-
- McPhee SJ, Bird JA. Implementation of cancer prevention guidelines in clinical practice. J Gen Intern Med 1990;(5 Suppl):S116–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

