Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs
- PMID: 15100402
- PMCID: PMC423214
- DOI: 10.1105/tpc.021030
Cotyledon vascular pattern2-mediated inositol (1,4,5) triphosphate signal transduction is essential for closed venation patterns of Arabidopsis foliar organs
Abstract
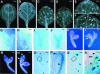
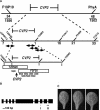
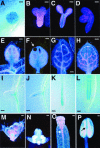
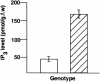
Vein patterns in leaves and cotyledons form in a spatially regulated manner through the progressive recruitment of ground cells into vascular cell fate. To gain insight into venation patterning mechanisms, we have characterized the cotyledon vascular pattern2 (cvp2) mutants, which exhibit an increase in free vein endings and a resulting open vein network. We cloned CVP2 by a map-based cloning strategy and found that it encodes an inositol polyphosphate 5' phosphatase (5PTase). 5PTases regulate inositol (1,4,5) triphosphate (IP(3)) signal transduction by hydrolyzing IP(3) and thus terminate IP(3) signaling. CVP2 gene expression is initially broad and then gradually restricted to incipient vascular cells in several developing organs. Consistent with the inferred enzymatic activity of CVP2, IP(3) levels are elevated in cvp2 mutants. In addition, cvp2 mutants exhibit hypersensitivity to the plant hormone abscisic acid. We propose that elevated IP(3) levels in cvp2 mutants reduce ground cell recruitment into vascular cell fate, resulting in premature vein termination and, thus, in an open reticulum.
Figures

References
-
- Allen, G.J., Chu, S.P., Harrington, C.L., Schumacher, K., Hoffmann, T., Tang, Y.Y., Grill, E., and Schroeder, J.I. (2001). A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411, 1053–1057. - PubMed
-
- Baima, S., Nobili, F., Sessa, G., Lucchetti, S., Ruberti, I., and Morelli, G. (1995). The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development 121, 4171–4182. - PubMed
-
- Berleth, T., and Jurgens, G. (1993). The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development 118, 575–587.
-
- Berridge, M.J. (1993). Inositol trisphosphate and calcium signalling. Nature 361, 315–325. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

