Changes in corticothalamic modulation of receptive fields during peripheral injury-induced reorganization
- PMID: 15100406
- PMCID: PMC406478
- DOI: 10.1073/pnas.0307840101
Changes in corticothalamic modulation of receptive fields during peripheral injury-induced reorganization
Abstract
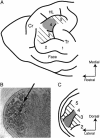
The influence of corticothalamic projections on the thalamus during different stages of reorganization was determined in anesthetized raccoons that had undergone previous removal of a single forepaw digit. Single-unit recordings were made from 522 sites in the somatosensory nucleus of the thalamus (ventroposterior lateral nucleus) before and after lesioning parts of primary somatosensory cortex. In those parts of ventroposterior lateral nucleus that had intact input from the periphery, the cortical lesion resulted in an immediate 85% increase in receptive field (RF) size. In animals studied 2-6 weeks after digit amputation, peripherally denervated thalamic neurons had unique RFs that were larger than normal, and these were not further enlarged by cortical lesion. However, at longer periods of reorganization (>4 mo), when the new RFs of denervated neurons had decreased in size, cortical lesion again produced expansion of RF size. These data demonstrate that corticothalamic fibers modulate the spatial extent of thalamic RFs in intact animals, probably by controlling intrathalamic inhibition. This corticothalamic modulation is ineffective during the early stages of injury-induced reorganization when new RFs are being formed, but is reinstated after the new RFs have become stabilized. The fact that neurons in the denervated thalamic region retained their unique RFs after cortical lesion indicates that their new inputs are not being relayed from a reorganized cortex and support the view that some plasticity occurs in or below the thalamus.
Figures


Similar articles
-
Changes in the organization of the ventroposterior lateral thalamic nucleus after digit removal in adult raccoon.J Comp Neurol. 1996 Jan 1;364(1):92-103. doi: 10.1002/(SICI)1096-9861(19960101)364:1<92::AID-CNE8>3.0.CO;2-N. J Comp Neurol. 1996. PMID: 8789278
-
The immediate effects of peripheral denervation on inhibitory mechanisms in the somatosensory thalamus.Somatosens Mot Res. 1993;10(1):69-80. doi: 10.3109/08990229309028825. Somatosens Mot Res. 1993. PMID: 8484298
-
Time-dependent changes in the functional organization of somatosensory cerebral cortex following digit amputation in adult raccoons.Somatosens Res. 1984;2(1):49-81. Somatosens Res. 1984. PMID: 6505463
-
Short-term functional plasticity of cortical and thalamic sensory representations and its implication for information processing.Adv Neurol. 1997;73:159-78. Adv Neurol. 1997. PMID: 8959213 Review.
-
Cortical and subcortical contributions to activity-dependent plasticity in primate somatosensory cortex.Annu Rev Neurosci. 2000;23:1-37. doi: 10.1146/annurev.neuro.23.1.1. Annu Rev Neurosci. 2000. PMID: 10845057 Review.
Cited by
-
AMPA and GABA(A/B) receptor subunit expression in the cuneate nucleus of adult squirrel monkeys during peripheral nerve regeneration.Neurosci Lett. 2014 Jan 24;559:141-6. doi: 10.1016/j.neulet.2013.11.054. Epub 2013 Dec 6. Neurosci Lett. 2014. PMID: 24315976 Free PMC article.
-
Impact of peripheral hearing loss on top-down auditory processing.Hear Res. 2017 Jan;343:4-13. doi: 10.1016/j.heares.2016.05.018. Epub 2016 May 31. Hear Res. 2017. PMID: 27260270 Free PMC article. Review.
References
-
- Steriade, M., Jones, E. G. & McCormick, D. A. (1997) Thalamus (Elsevier, Amsterdam).
-
- Tsumoto, T., Creutzfeldt, O. D. & Legendy, C. R. (1978) Exp. Brain Res. 32, 345-364. - PubMed
-
- Zhang, Y. & Suga, N. (1997) J. Neurophysiol. 78, 3489-3492. - PubMed
-
- Zhang, Y., Suga, N. & Yan, J. (1997) Nature 387, 900-903. - PubMed
-
- Rapisarda, C., Palmeri, A. & Sapienza, S. (1992) Exp. Brain Res. 88, 140-150. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

