A single polypyrimidine tract binding protein (PTB) binding site mediates splicing inhibition at mouse IgM exons M1 and M2
- PMID: 15100434
- PMCID: PMC1370569
- DOI: 10.1261/rna.5229704
A single polypyrimidine tract binding protein (PTB) binding site mediates splicing inhibition at mouse IgM exons M1 and M2
Abstract
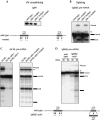
Splicing of mouse immunoglobulin (IgM) exons M1 and M2 is directed by two juxtaposed regulatory elements, an enhancer and an inhibitor, located within the M2 exon. A primary function of the enhancer is to counteract the inhibitor, allowing splicing to occur. Here we show that the inhibitor contains two binding sites for polypyrimidine tract binding protein (PTB). Mutational analysis indicates that only one of these sites is necessary and sufficient to direct splicing inhibition both in vitro and in vivo. We demonstrate that the difference in activity of the two sites is explained by proximity to the intron. We further show that the presence of the enhancer results in the disruption of the PTB-inhibitor interaction, enabling splicing to occur. In the absence of the enhancer, splicing can be artificially activated by immuno-inhibition of PTB. Collectively, our results indicate that a single PTB binding site can function as an inhibitor that regulates alternative splicing both in vitro and in vivo.
Figures


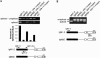
Similar articles
-
An intron enhancer recognized by splicing factors activates polyadenylation.Genes Dev. 1996 Jan 15;10(2):208-19. doi: 10.1101/gad.10.2.208. Genes Dev. 1996. PMID: 8566754
-
Polypyrimidine tract binding protein inhibits IgM pre-mRNA splicing by diverting U2 snRNA base-pairing away from the branch point.RNA. 2014 Apr;20(4):440-6. doi: 10.1261/rna.043737.113. Epub 2014 Feb 26. RNA. 2014. PMID: 24572809 Free PMC article.
-
Exon repression by polypyrimidine tract binding protein.RNA. 2005 May;11(5):699-716. doi: 10.1261/rna.2250405. RNA. 2005. PMID: 15840818 Free PMC article.
-
Regulation of alternative splicing by PTB and associated factors.Biochem Soc Trans. 2005 Jun;33(Pt 3):457-60. doi: 10.1042/BST0330457. Biochem Soc Trans. 2005. PMID: 15916540 Review.
-
New insights into functional roles of the polypyrimidine tract-binding protein.Int J Mol Sci. 2013 Nov 20;14(11):22906-32. doi: 10.3390/ijms141122906. Int J Mol Sci. 2013. PMID: 24264039 Free PMC article. Review.
Cited by
-
Polypyrimidine tract binding protein (hnRNP I) is possibly a conserved modulator of miRNA-mediated gene regulation.PLoS One. 2012;7(3):e33144. doi: 10.1371/journal.pone.0033144. Epub 2012 Mar 9. PLoS One. 2012. PMID: 22427970 Free PMC article.
-
Repression of alpha-actinin SM exon splicing by assisted binding of PTB to the polypyrimidine tract.RNA. 2007 Aug;13(8):1214-23. doi: 10.1261/rna.219607. Epub 2007 Jun 25. RNA. 2007. PMID: 17592047 Free PMC article.
-
Functional coupling of RNAP II transcription to spliceosome assembly.Genes Dev. 2006 May 1;20(9):1100-9. doi: 10.1101/gad.1397406. Genes Dev. 2006. PMID: 16651655 Free PMC article.
-
Exonic splicing enhancers in fission yeast: functional conservation demonstrates an early evolutionary origin.Genes Dev. 2005 Jan 15;19(2):242-54. doi: 10.1101/gad.1265905. Epub 2004 Dec 29. Genes Dev. 2005. PMID: 15625190 Free PMC article.
-
The RNA polymerase II C-terminal domain promotes splicing activation through recruitment of a U2AF65-Prp19 complex.Genes Dev. 2011 May 1;25(9):972-83. doi: 10.1101/gad.2038011. Genes Dev. 2011. PMID: 21536736 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
