Matrix remodeling during endochondral ossification
- PMID: 15102440
- PMCID: PMC2779708
- DOI: 10.1016/j.tcb.2003.12.003
Matrix remodeling during endochondral ossification
Abstract
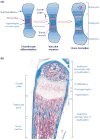
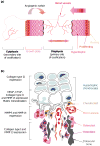
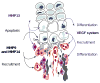
Endochondral ossification, the process by which most of the skeleton is formed, is a powerful system for studying various aspects of the biological response to degraded extracellular matrix (ECM). In addition, the dependence of endochondral ossification upon neovascularization and continuous ECM remodeling provides a good model for studying the role of the matrix metalloproteases (MMPs) not only as simple effectors of ECM degradation but also as regulators of active signal-inducers for the initiation of endochondral ossification. The daunting task of elucidating their specific role during endochondral ossification has been facilitated by the development of mice deficient for various members of this family. Here, we discuss the ECM and its remodeling as one level of molecular regulation for the process of endochondral ossification, with special attention to the MMPs.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

