Cost effectiveness analysis of neonatal extracorporeal membrane oxygenation based on four year results from the UK Collaborative ECMO Trial
- PMID: 15102733
- PMCID: PMC1721674
- DOI: 10.1136/adc.2002.025635
Cost effectiveness analysis of neonatal extracorporeal membrane oxygenation based on four year results from the UK Collaborative ECMO Trial
Abstract
Objective: To assess the cost effectiveness of extracorporeal membrane oxygenation (ECMO) for mature newborn infants with severe respiratory failure over a four year time span.
Design: Cost effectiveness analysis based on a randomised controlled trial in which infants were individually allocated to ECMO (intervention) or conventional management (control) and then followed up to 4 years of age.
Setting: Infants were recruited from 55 approved recruiting hospitals throughout the United Kingdom. Infants allocated to ECMO were transferred to one of five specialist regional centres. Follow up of surviving infants was performed in the community.
Subjects: A total of 185 mature (gestational age at birth >or= 35 weeks, birth weight >or= 2000 g) newborn infants with severe respiratory failure (oxygenation index >or= 40).
Main outcome measures: Incremental cost per additional life year gained; incremental cost per additional disability-free life year gained.
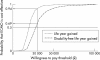
Results: Over four years, the policy of neonatal ECMO was effective at reducing known death or severe disability (relative risk = 0.64; 95% confidence interval 0.47 to 0.86; p = 0.004). After adjustment for censoring and discounting at 6%, the mean additional health service cost of neonatal ECMO was pound 17367 (95% confidence interval pound 12072 to pound 22224) per infant ( pound UK, 2001 prices). Over four years, the incremental cost of neonatal ECMO was pound 16707 ( pound 9828 to pound 37924) per life year gained and pound 24775 ( pound 13106 to pound 69690) per disability-free life year gained. These results remained robust after variations in the values of key variables performed as part of a sensitivity analysis.
Conclusions: The study provides rigorous evidence of the cost effectiveness of ECMO at four years for mature infants with severe respiratory failure.
Figures
Similar articles
-
Cost-effectiveness of neonatal extracorporeal membrane oxygenation based on 7-year results from the United Kingdom Collaborative ECMO Trial.Pediatrics. 2006 May;117(5):1640-9. doi: 10.1542/peds.2005-1150. Pediatrics. 2006. PMID: 16651318 Clinical Trial.
-
UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK Collaborative ECMO Trail Group.Lancet. 1996 Jul 13;348(9020):75-82. Lancet. 1996. PMID: 8676720 Clinical Trial.
-
Economic evaluation and randomised controlled trial of extracorporeal membrane oxygenation: UK collaborative trial. The Extracorporeal Membrane Oxygenation Economics Working Group.BMJ. 1998 Oct 3;317(7163):911-5; discussion 915-6. doi: 10.1136/bmj.317.7163.911. BMJ. 1998. PMID: 9756807 Free PMC article. Clinical Trial.
-
The current status of neonatal extracorporeal membrane oxygenation.Semin Perinatol. 2000 Dec;24(6):406-17. doi: 10.1053/sper.2000.20086. Semin Perinatol. 2000. PMID: 11153902 Review.
-
Current status of extracorporeal membrane oxygenation for severe respiratory failure.Artif Organs. 1996 Feb;20(2):120-3. doi: 10.1111/j.1525-1594.1996.tb00712.x. Artif Organs. 1996. PMID: 8712954 Review.
Cited by
-
Methods of data collection and analysis for the economic evaluation alongside a national, multi-centre trial in the UK: conventional ventilation or ECMO for Severe Adult Respiratory Failure (CESAR).BMC Health Serv Res. 2008 Apr 30;8:94. doi: 10.1186/1472-6963-8-94. BMC Health Serv Res. 2008. PMID: 18447931 Free PMC article. Clinical Trial.
-
Extracorporeal membrane oxygenation in children: A brief review.J Paediatr Child Health. 2022 Sep;58(9):1525-1531. doi: 10.1111/jpc.16151. Epub 2022 Aug 6. J Paediatr Child Health. 2022. PMID: 35932281 Free PMC article. Review.
-
Transportation of patients on extracorporeal membrane oxygenation: a tertiary medical center experience and systematic review of the literature.Ann Intensive Care. 2017 Dec;7(1):14. doi: 10.1186/s13613-016-0232-7. Epub 2017 Feb 7. Ann Intensive Care. 2017. PMID: 28176223 Free PMC article.
-
Successful Establishment of the First Neonatal Respiratory Extracorporeal Membrane Oxygenation (ECMO) Program in the Middle East, in Collaboration With Pediatric Services.Front Pediatr. 2020 Sep 11;8:506. doi: 10.3389/fped.2020.00506. eCollection 2020. Front Pediatr. 2020. PMID: 33014924 Free PMC article.
-
Features of randomised trials designed by the NPEU Perinatal Trials Service during Adrian Grant's directorship.Reprod Health. 2018 Jul 9;15(1):125. doi: 10.1186/s12978-018-0567-7. Reprod Health. 2018. PMID: 29986758 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources