Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections
- PMID: 15102757
- PMCID: PMC387888
- DOI: 10.1128/IAI.72.5.2507-2512.2004
Intranasal immunization with multivalent group A streptococcal vaccines protects mice against intranasal challenge infections
Abstract
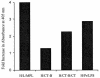
We have previously shown that a hexavalent group A streptococcal M protein-based vaccine evoked bactericidal antibodies after intramuscular injection. In the present study, we show that the hexavalent vaccine formulated with several different mucosal adjuvants and delivered intranasally induced serum and salivary antibodies that protected mice from intranasal challenge infections with virulent group A streptococci. The hexavalent vaccine was formulated with liposomes with or without monophosphorylated lipid A (MPL), cholera toxin B subunit with or without holotoxin, or proteosomes from Neisseria meningitidis outer membrane proteins complexed with lipopolysaccharide from Shigella flexneri. Intranasal immunization with the hexavalent vaccine mixed with these adjuvants resulted in significant levels of antibodies in serum 2 weeks after the final dose. Mean serum antibody titers were equivalent in all groups of mice except those that were immunized with hexavalent protein plus liposomes without MPL, which were significantly lower. Salivary antibodies were also detected in mice that received the vaccine formulated with the four strongest adjuvants. T-cell proliferative assays and cytokine assays using lymphocytes from cervical lymph nodes and spleens from mice immunized with the hexavalent vaccine formulated with proteosomes indicated the presence of hexavalent protein-specific T cells and a Th1-weighted mixed Th1-Th2 cytokine profile. Intranasal immunization with adjuvanted formulations of the hexavalent vaccine resulted in significant levels of protection (80 to 100%) following intranasal challenge infections with type 24 group A streptococci. Our results indicate that intranasal delivery of adjuvanted multivalent M protein vaccines induces protective antibody responses and may provide an alternative to parenteral vaccine formulations.
Figures
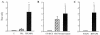

Similar articles
-
Protective immunity induced by an intranasal multivalent vaccine comprising 10 Lactococcus lactis strains expressing highly prevalent M-protein antigens derived from Group A Streptococcus.Microbiol Immunol. 2018 Jun;62(6):395-404. doi: 10.1111/1348-0421.12595. Epub 2018 Jun 11. Microbiol Immunol. 2018. PMID: 29704396 Free PMC article.
-
Prime-Pull Immunization with a Bivalent M-Protein and Spy-CEP Peptide Vaccine Adjuvanted with CAF®01 Liposomes Induces Both Mucosal and Peripheral Protection from covR/S Mutant Streptococcus pyogenes.mBio. 2021 Feb 23;12(1):e03537-20. doi: 10.1128/mBio.03537-20. mBio. 2021. PMID: 33622722 Free PMC article.
-
Intranasal administration is an effective mucosal vaccine delivery route for self-adjuvanting lipid core peptides targeting the group A streptococcal M protein.J Infect Dis. 2006 Aug 1;194(3):316-24. doi: 10.1086/505580. Epub 2006 Jun 30. J Infect Dis. 2006. PMID: 16826479
-
Update on group A streptococcal vaccine development.Curr Opin Infect Dis. 2020 Jun;33(3):244-250. doi: 10.1097/QCO.0000000000000644. Curr Opin Infect Dis. 2020. PMID: 32304470 Free PMC article. Review.
-
Nanotechnology in vaccine delivery.Adv Drug Deliv Rev. 2008 May 22;60(8):915-28. doi: 10.1016/j.addr.2007.05.017. Epub 2008 Feb 7. Adv Drug Deliv Rev. 2008. PMID: 18325628 Free PMC article. Review.
Cited by
-
Disease manifestations and pathogenic mechanisms of Group A Streptococcus.Clin Microbiol Rev. 2014 Apr;27(2):264-301. doi: 10.1128/CMR.00101-13. Clin Microbiol Rev. 2014. PMID: 24696436 Free PMC article. Review.
-
Local Th17/IgA immunity correlate with protection against intranasal infection with Streptococcus pyogenes.PLoS One. 2017 Apr 17;12(4):e0175707. doi: 10.1371/journal.pone.0175707. eCollection 2017. PLoS One. 2017. PMID: 28414746 Free PMC article.
-
Immunotherapy targeting the Streptococcus pyogenes M protein or streptolysin O to treat or prevent influenza A superinfection.PLoS One. 2020 Jun 23;15(6):e0235139. doi: 10.1371/journal.pone.0235139. eCollection 2020. PLoS One. 2020. PMID: 32574205 Free PMC article.
-
Streptococcal protective antigens (Spa): a new family of type-specific proteins of group A streptococci.Eur J Clin Microbiol Infect Dis. 2010 Jan;29(1):51-7. doi: 10.1007/s10096-009-0819-0. Epub 2009 Oct 29. Eur J Clin Microbiol Infect Dis. 2010. PMID: 19865839
-
The prospect of vaccination against group A beta-hemolytic streptococci.Curr Infect Dis Rep. 2008 May;10(3):192-9. doi: 10.1007/s11908-008-0032-9. Curr Infect Dis Rep. 2008. PMID: 18510880
References
-
- Alving, C. R. 1991. Liposomes as carriers of antigens and adjuvants. J. Immunol. Methods 140:1-13. - PubMed
-
- Beachey, E. H., M. Bronze, J. B. Dale, W. Kraus, T. Poirier, and S. Sargent. 1988. Protective and autoimmune epitopes of streptococcal M proteins. Vaccine 6:192-196. - PubMed
-
- Bisno, A., I. Pearce, H. Wall, M. Moody, and G. Stollerman. 1970. Contrasting epidemiology of acute rheumatic fever and acute glomerulonephritis: nature of the antecedent streptococcal infection. N. Engl. J. Med. 283:561-565. - PubMed
-
- Brandt, E. R., W. A. Hayman, B. Currie, S. Pruksakorn, and M. F. Good. 1997. Human antibodies to the conserved region of the M protein: opsonization of heterologous strains of group A streptococci. Vaccine 15:1805-1812. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

