Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection
- PMID: 15102765
- PMCID: PMC387854
- DOI: 10.1128/IAI.72.5.2574-2581.2004
Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection
Abstract
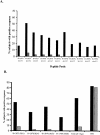
The tuberculin skin test for diagnosing Mycobacterium tuberculosis infection suffers from antigenic cross-reactivity of purified protein derivative with BCG, resulting in poor specificity in BCG-vaccinated populations. Comparative genomics has identified several genetic regions in M. tuberculosis and M. bovis that are deleted in M. bovis BCG. Proteins encoded in these regions will form the basis of new specific T-cell-based blood tests that do not cross-react with BCG, but only two, early secretory antigen target 6 and culture filtrate protein 10, have been studied in detail in humans. We investigated four novel gene products, encoded by RD2 (Rv1989c) and RD1 (Rv3873, Rv3878, and Rv3879c), that are absent from most or all of the vaccine strains of BCG, respectively. Sixty-seven overlapping peptides were tested in ex vivo gamma interferon enzyme-linked immunospot assays in 49 patients with culture-confirmed tuberculosis and 38 healthy BCG-vaccinated donors. Forty-five percent (95% confidence interval [CI], 31 to 57%) and 53% (95% CI, 39 to 67%) of the tuberculosis patients responded to Rv3879c and Rv3873, respectively, identifying these proteins as major M. tuberculosis T-cell antigens in humans, while 35 and 25% of the patients responded to Rv3878 and Rv1989c, respectively. Of the 38 BCG-vaccinated donors, 1 (2.6%) responded to peptides from Rv3878 and Rv3879c, 3 (7.9%) responded to Rv3873, and none responded to Rv1989c. Exclusion of cross-reactive peptides encoded in conserved motifs of Rv3873, a PPE family member, increased its specificity to 97.4%. The high specificity of Rv3879c peptides and nonconserved Rv3873 sequences, together with their moderate sensitivity in tuberculosis patients, identifies these peptides as candidates for inclusion in new T-cell-based tests for M. tuberculosis infection.
Figures

Similar articles
-
[Application of T cell responses to novel RD1-encoded mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008 Jun;30(3):305-8. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2008. PMID: 18686610 Chinese.
-
PPE protein (Rv3873) from DNA segment RD1 of Mycobacterium tuberculosis: strong recognition of both specific T-cell epitopes and epitopes conserved within the PPE family.Infect Immun. 2003 Nov;71(11):6116-23. doi: 10.1128/IAI.71.11.6116-6123.2003. Infect Immun. 2003. PMID: 14573626 Free PMC article.
-
Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics.Infect Immun. 2002 Dec;70(12):6996-7003. doi: 10.1128/IAI.70.12.6996-7003.2002. Infect Immun. 2002. PMID: 12438379 Free PMC article.
-
[Evolution of IGRA researches].Kekkaku. 2008 Sep;83(9):641-52. Kekkaku. 2008. PMID: 18979999 Review. Japanese.
-
Alternatives to the tuberculin skin test: interferon-gamma assays in the diagnosis of mycobacterium tuberculosis infection.Indian J Med Microbiol. 2005 Jul;23(3):151-8. doi: 10.4103/0255-0857.16585. Indian J Med Microbiol. 2005. PMID: 16100419 Review.
Cited by
-
BCG vaccine in Korea.Clin Exp Vaccine Res. 2013 Jul;2(2):83-91. doi: 10.7774/cevr.2013.2.2.83. Epub 2013 Jul 3. Clin Exp Vaccine Res. 2013. PMID: 23858398 Free PMC article.
-
Mycobacterium tuberculosis region of difference (RD) 2 antigen Rv1985c and RD11 antigen Rv3425 have the promising potential to distinguish patients with active tuberculosis from M. bovis BCG-vaccinated individuals.Clin Vaccine Immunol. 2013 Jan;20(1):69-76. doi: 10.1128/CVI.00481-12. Epub 2012 Nov 7. Clin Vaccine Immunol. 2013. PMID: 23136116 Free PMC article.
-
Prevalence of Latent Tuberculosis Infection among Medical Students in South Korea.Tuberc Respir Dis (Seoul). 2012 Oct;73(4):219-23. doi: 10.4046/trd.2012.73.4.219. Epub 2012 Oct 31. Tuberc Respir Dis (Seoul). 2012. PMID: 23166557 Free PMC article.
-
Evaluation of Rv0220, Rv2958c, Rv2994 and Rv3347c of Mycobacterium tuberculosis for serodiagnosis of tuberculosis.Microb Biotechnol. 2017 May;10(3):604-611. doi: 10.1111/1751-7915.12697. Epub 2017 Feb 20. Microb Biotechnol. 2017. PMID: 28217905 Free PMC article.
-
Serodiagnosis efficacy and immunogenicity of the fusion protein of Mycobacterium tuberculosis composed of the 10-kilodalton culture filtrate protein, ESAT-6, and the extracellular domain fragment of PPE68.Clin Vaccine Immunol. 2012 Apr;19(4):536-44. doi: 10.1128/CVI.05708-11. Epub 2012 Feb 22. Clin Vaccine Immunol. 2012. PMID: 22357648 Free PMC article.
References
-
- American Thoracic Society and Centers for Disease Control and Prevention. 2000. Targeted tuberculin testing and treatment of latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 161:S221-S247. - PubMed
-
- Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. - PubMed
-
- Andersen, P., M. E. Munk, J. M. Pollock, and T. M. Doherty. 2000. Specific immune-based diagnosis of tuberculosis. Lancet 356:1099-1104. - PubMed
-
- Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. - PubMed
-
- Arend, S. M., A. C. Engelhard, G. Groot, K. de Boer, P. Andersen, T. H. Ottenhoff, and J. T. van Dissel. 2001. Tuberculin skin testing compared with T-cell responses to Mycobacterium tuberculosis-specific and nonspecific antigens for detection of latent infection in persons with recent tuberculosis contact. Clin. Diagn. Lab. Immunol. 8:1089-1096. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

