Caspase-8 activation precedes alterations of mitochondrial membrane potential during monocyte apoptosis induced by phagocytosis and killing of Staphylococcus aureus
- PMID: 15102767
- PMCID: PMC387870
- DOI: 10.1128/IAI.72.5.2590-2597.2004
Caspase-8 activation precedes alterations of mitochondrial membrane potential during monocyte apoptosis induced by phagocytosis and killing of Staphylococcus aureus
Abstract
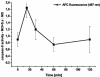
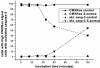
Human peripheral blood monocytes become apoptotic following phagocytosis and killing of Staphylococcus aureus. Although this type of monocyte apoptosis is known to be initiated by Fas-Fas ligand (FasL) interactions, the downstream signaling pathway has not been determined. In this work the involvement of mitochondria and the kinetics of caspase-8 and caspase-3 activation after phagocytosis of S. aureus were studied. Caspase-8 activity was measured in cell lysates by using the fluorogenic substrate Ac-IETD-AFC. Active caspase-3 levels and mitochondrial membrane potential (Deltapsi(m)) were measured in whole cells by flow cytometry using monoclonal antibodies reacting with activated caspase-3 and chloromethyl-X-rosamine, respectively. The results show that caspase-8 was activated shortly after phagocytosis of bacteria. Caspase-8 activation was followed by progressive disruption of Deltapsi(m), which is associated with the production of reactive oxygen intermediates. The irreversible caspase-8 inhibitor zIETD-FMK prevented the disruption of Deltapsi(m) and the release of cytochrome c from S. aureus-exposed monocytes. Caspase-3 activation occurred following disruption of Deltapsi(m). These results strongly suggest that apoptosis of monocytes that have phagocytosed and killed S. aureus is driven by the Fas-FasL-initiated pathway, which is typical for type II cells.
Figures




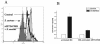
Similar articles
-
Fas (CD95)-Fas ligand interactions are responsible for monocyte apoptosis occurring as a result of phagocytosis and killing of Staphylococcus aureus.Infect Immun. 2001 Mar;69(3):1287-97. doi: 10.1128/IAI.69.3.1287-1297.2001. Infect Immun. 2001. PMID: 11179290 Free PMC article.
-
Involvement of mitochondria and caspase-3 in ET-18-OCH(3)-induced apoptosis of human leukemic cells.Int J Cancer. 2000 Apr 15;86(2):208-18. doi: 10.1002/(sici)1097-0215(20000415)86:2<208::aid-ijc10>3.0.co;2-e. Int J Cancer. 2000. PMID: 10738248
-
alpha-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling.J Cell Biol. 2001 Nov 12;155(4):637-48. doi: 10.1083/jcb.200105081. Epub 2001 Nov 5. J Cell Biol. 2001. PMID: 11696559 Free PMC article.
-
The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction.Cell Death Differ. 2003 Mar;10(3):323-34. doi: 10.1038/sj.cdd.4401148. Cell Death Differ. 2003. PMID: 12700632 Free PMC article.
-
Synthetic 1,4-anthracenedione analogs induce cytochrome c release, caspase-9, -3, and -8 activities, poly(ADP-ribose) polymerase-1 cleavage and internucleosomal DNA fragmentation in HL-60 cells by a mechanism which involves caspase-2 activation but not Fas signaling.Biochem Pharmacol. 2004 Feb 1;67(3):523-37. doi: 10.1016/j.bcp.2003.09.012. Biochem Pharmacol. 2004. PMID: 15037204
Cited by
-
Death receptor signals to mitochondria.Cancer Biol Ther. 2004 Nov;3(11):1051-7. doi: 10.4161/cbt.3.11.1173. Epub 2004 Nov 18. Cancer Biol Ther. 2004. PMID: 15640619 Free PMC article. Review.
-
A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages.PLoS One. 2008 Jan 9;3(1):e1409. doi: 10.1371/journal.pone.0001409. PLoS One. 2008. PMID: 18183290 Free PMC article.
-
Regulation of Apoptosis by Gram-Positive Bacteria: Mechanistic Diversity and Consequences for Immunity.Curr Immunol Rev. 2006 May;2(2):119-141. doi: 10.2174/157339506776843033. Curr Immunol Rev. 2006. PMID: 19081777 Free PMC article.
-
Human neuroblastoma cells rapidly enter cell cycle arrest and apoptosis following exposure to C-28 derivatives of the synthetic triterpenoid CDDO.Cancer Biol Ther. 2008 May;7(5):709-17. doi: 10.4161/cbt.7.5.5713. Epub 2008 May 7. Cancer Biol Ther. 2008. PMID: 18277094 Free PMC article.
-
Staphylococcal PknB as the first prokaryotic representative of the proline-directed kinases.PLoS One. 2010 Feb 4;5(2):e9057. doi: 10.1371/journal.pone.0009057. PLoS One. 2010. PMID: 20140229 Free PMC article.
References
-
- Adrain, C., E. M. Creagh, and S. J. Martin. 2002. Caspase cascades in apoptosis, p. 41-51. In M. Los and H. Walczak (ed.), Caspases: their role in cell death and cell survival. Kluwer Academic/Plenum Publishers, New York, N.Y.
-
- Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

