Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis
- PMID: 15102771
- PMCID: PMC387880
- DOI: 10.1128/IAI.72.5.2628-2634.2004
Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis
Abstract
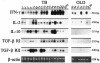
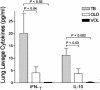
Immune factors influencing progression to active tuberculosis (TB) remain poorly defined. In this study, we investigated the expression of immunoregulatory cytokines and receptors by using lung bronchoalveolar lavage cells obtained from patients with pulmonary TB, patients with other lung diseases (OLD patients), and healthy volunteers (VOL) by using reverse transcriptase PCR, a transforming growth factor beta (TGF-beta) bioactivity assay, and an enzyme immunoassay. TB patients were significantly more likely than OLD patients to coexpress TGF-beta receptor I (RI) and RII mRNA, as well as interleukin-10 (IL-10) mRNA (thereby indicating the state of active gene transcription in the alveolar cells at harvest). In contrast, gamma interferon (IFN-gamma) and IL-2 mRNA was seen in both TB and OLD patients. Likewise, significantly elevated pulmonary steady-state protein levels of IL-10, IFN-gamma, and bioactive TGF-beta were found in TB patients versus those in OLD patients and VOL. These data suggest that the combined production of the immunosuppressants IL-10 and TGF-beta, as well as coexpression of TGF-beta RI and RII (required for cellular response to TGF-beta), may act to down-modulate host anti-Mycobacterium tuberculosis immunity and thereby allow uncontrolled bacterial replication and overt disease. Delineating the underlying mechanisms of M. tuberculosis-triggered expression of these immune elements may provide a molecular-level understanding of TB immunopathogenesis.
Figures

Similar articles
-
Reduction in transforming growth factor-beta type II receptor in mouse lung carcinogenesis.Mol Carcinog. 1998 May;22(1):46-56. doi: 10.1002/(sici)1098-2744(199805)22:1<46::aid-mc6>3.0.co;2-j. Mol Carcinog. 1998. PMID: 9609100
-
Enhanced tumorigenesis and reduced transforming growth factor-beta type II receptor in lung tumors from mice with reduced gene dosage of transforming growth factor-beta1.Mol Carcinog. 2000 Oct;29(2):112-26. doi: 10.1002/1098-2744(200010)29:2<112::aid-mc8>3.0.co;2-9. Mol Carcinog. 2000. PMID: 11074608
-
Human pulmonary acinar aplasia: reduction of transforming growth factor-beta ligands and receptors.Pediatr Res. 1999 Jul;46(1):61-70. doi: 10.1203/00006450-199907000-00011. Pediatr Res. 1999. PMID: 10400136
-
TGF-betas and TGF-beta receptors in atherosclerosis.Cytokine Growth Factor Rev. 2000 Mar-Jun;11(1-2):103-14. doi: 10.1016/s1359-6101(99)00034-9. Cytokine Growth Factor Rev. 2000. PMID: 10708958 Review.
-
Immunoregulation in TB: observations and implications.Clin Transl Sci. 2010 Feb;3(1):23-8. doi: 10.1111/j.1752-8062.2010.00180.x. Clin Transl Sci. 2010. PMID: 20443950 Free PMC article. Review.
Cited by
-
IL-10 Impairs Local Immune Response in Lung Granulomas and Lymph Nodes during Early Mycobacterium tuberculosis Infection.J Immunol. 2020 Feb 1;204(3):644-659. doi: 10.4049/jimmunol.1901211. Epub 2019 Dec 20. J Immunol. 2020. PMID: 31862711 Free PMC article.
-
Expression patterns and action analysis of genes associated with physiological responses during rat liver regeneration: cellular immune response.World J Gastroenterol. 2006 Dec 14;12(46):7514-21. doi: 10.3748/wjg.v12.i46.7514. World J Gastroenterol. 2006. PMID: 17167843 Free PMC article.
-
The Impact of Alcohol Use Disorder on Tuberculosis: A Review of the Epidemiology and Potential Immunologic Mechanisms.Front Immunol. 2022 Mar 31;13:864817. doi: 10.3389/fimmu.2022.864817. eCollection 2022. Front Immunol. 2022. PMID: 35432348 Free PMC article. Review.
-
Exploring the Role of Low-Density Neutrophils During Mycobacterium tuberculosis Infection.Front Cell Infect Microbiol. 2022 Jun 21;12:901590. doi: 10.3389/fcimb.2022.901590. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35800386 Free PMC article. Review.
-
Macrophage immunoregulatory pathways in tuberculosis.Semin Immunol. 2014 Dec;26(6):471-85. doi: 10.1016/j.smim.2014.09.010. Epub 2014 Oct 30. Semin Immunol. 2014. PMID: 25453226 Free PMC article. Review.
References
-
- Barnes, P. F., A. B. Bloch, P. T. Davidson, and D. E. Snider. 1991. Tuberculosis in patients with HIV infection. N. Engl. J. Med. 324:1644-1650. - PubMed
-
- Bonecini-Almeida, M. G., S. Chitale, I. Boutsikakis, J. Geng, H. Doo, S. He, and J. L. Ho. 1998. Induction of in vitro human macrophage anti-Mycobacterium tuberculosis activity: requirement for IFN-gamma and primed lymphocytes. J. Immunol. 160:4490-4499. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

