Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells
- PMID: 15102775
- PMCID: PMC387913
- DOI: 10.1128/IAI.72.5.2671-2678.2004
Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells
Abstract
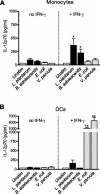
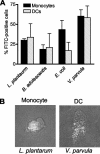
The normal gastrointestinal bacterial flora is crucial for the maturation of acquired immunity via effects on antigen-presenting cells (APCs). Here we investigated how two types of APCs, monocytes and dendritic cells (DCs), react to different bacterial strains typical of the commensal intestinal microflora. Purified human monocytes and monocyte-derived DCs were stimulated with UV-inactivated gram-positive (Lactobacillus plantarum and Bifidobacterium adolescentis) and gram-negative (Escherichia coli and Veillonella parvula) bacterial strains. Monocytes produced higher levels of interleukin 12p70 (IL-12p70) and tumor necrosis factor (TNF), as detected by an enzyme-linked immunosorbent assay, in response to L. plantarum than in response to E. coli and V. parvula. In contrast, DCs secreted large amounts of IL-12p70, TNF, IL-6, and IL-10 in response to E. coli and V. parvula but were practically unresponsive to L. plantarum and B. adolescentis. The lack of a response to the gram-positive strains correlated with lower surface expression of Toll-like receptor 2 (TLR2) on DCs than on monocytes. The surface expression of TLR4 on DCs was undetectable when it was analyzed by flow cytometry, but blocking this receptor decreased the TNF production in response to V. parvula, indicating that TLR4 is expressed at a low density on DCs. Gamma interferon increased the expression of TLR4 on DCs and also potentiated the cytokine response to the gram-negative strains. Our results indicate that when monocytes differentiate into DCs, their ability to respond to different commensal bacteria dramatically changes, and they become unresponsive to probiotic gram-positive bacteria. These results may have important implications for the abilities of different groups of commensal bacteria to regulate mucosal and systemic immunity.
Figures

Similar articles
-
Innate immune responses of human neonatal cells to bacteria from the normal gastrointestinal flora.Infect Immun. 2002 Dec;70(12):6688-96. doi: 10.1128/IAI.70.12.6688-6696.2002. Infect Immun. 2002. PMID: 12438343 Free PMC article.
-
IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities.J Immunol. 2003 Oct 1;171(7):3385-93. doi: 10.4049/jimmunol.171.7.3385. J Immunol. 2003. PMID: 14500632
-
Differential production of cytokines, reactive oxygen and nitrogen by bovine macrophages and dendritic cells stimulated with Toll-like receptor agonists.Immunology. 2004 Jan;111(1):41-52. doi: 10.1111/j.1365-2567.2004.01781.x. Immunology. 2004. PMID: 14678198 Free PMC article.
-
Role of MD-2 in TLR2- and TLR4-mediated recognition of Gram-negative and Gram-positive bacteria and activation of chemokine genes.J Endotoxin Res. 2000;6(5):401-5. doi: 10.1179/096805100101532243. J Endotoxin Res. 2000. PMID: 11521063 Review.
-
Dendritic cell subsets in the intestinal lamina propria: ontogeny and function.Eur J Immunol. 2013 Dec;43(12):3098-107. doi: 10.1002/eji.201343740. Epub 2013 Aug 21. Eur J Immunol. 2013. PMID: 23966272 Free PMC article. Review.
Cited by
-
Stronger T cell immunogenicity of ovalbumin expressed intracellularly in Gram-negative than in Gram-positive bacteria.PLoS One. 2013 May 31;8(5):e65124. doi: 10.1371/journal.pone.0065124. Print 2013. PLoS One. 2013. PMID: 23741469 Free PMC article.
-
Soluble CD14 and CD83 from human neonatal antigen-presenting cells are inducible by commensal bacteria and suppress allergen-induced human neonatal Th2 differentiation.Infect Immun. 2007 Aug;75(8):4097-104. doi: 10.1128/IAI.01744-06. Epub 2007 May 25. Infect Immun. 2007. PMID: 17526743 Free PMC article.
-
Well-controlled proinflammatory cytokine responses of Peyer's patch cells to probiotic Lactobacillus casei.Immunology. 2010 Jul;130(3):352-62. doi: 10.1111/j.1365-2567.2009.03204.x. Immunology. 2010. PMID: 20636824 Free PMC article.
-
Immunobiotic Lactobacillus jensenii modulates the Toll-like receptor 4-induced inflammatory response via negative regulation in porcine antigen-presenting cells.Clin Vaccine Immunol. 2012 Jul;19(7):1038-53. doi: 10.1128/CVI.00199-12. Epub 2012 May 9. Clin Vaccine Immunol. 2012. PMID: 22573738 Free PMC article.
-
Genes and molecules of lactobacilli supporting probiotic action.Microbiol Mol Biol Rev. 2008 Dec;72(4):728-64, Table of Contents. doi: 10.1128/MMBR.00017-08. Microbiol Mol Biol Rev. 2008. PMID: 19052326 Free PMC article. Review.
References
-
- Adlerberth, I., L. Å. Hansson, and A. E. Wold. 2000. Ontogeny of the intestinal flora, p. 279-292. In I. R. Sanderson and W. A. Walker (ed.), Development of the gastrointestinal tract. BC Decker Inc., Hamilton, Ontario, Canada.
-
- Agrawal, S., A. Agrawal, B. Doughty, A. Gerwitz, J. Blenis, T. Van Dyke, and B. Pulendran. 2003. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J. Immunol. 171:4984-4989. - PubMed
-
- Ahrne, S., S. Nobaek, B. Jeppsson, I. Adlerberth, A. E. Wold, and G. Molin. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. - PubMed
-
- Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. - PubMed
-
- Albers, W. H., C. W. Tyler, and B. Boxerbaum. 1966. Asymptomatic bacteremia in the newborn infant. J. Pediatr. 69:193-197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

