Intranasal immunization with a colloid-formulated bacterial extract induces an acute inflammatory response in the lungs and elicits specific immune responses
- PMID: 15102776
- PMCID: PMC387843
- DOI: 10.1128/IAI.72.5.2679-2688.2004
Intranasal immunization with a colloid-formulated bacterial extract induces an acute inflammatory response in the lungs and elicits specific immune responses
Abstract
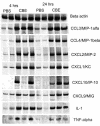
Nonspecific stimulation of lung defenses by repeated oral administration of immunomodulators, such as bacterial extracts, has shown potential for the prevention of respiratory tract infections. Here, we show that intranasal (i.n.) immunization with a bacterial extract formulated as a colloid induces an acute inflammatory response in the lungs characterized by increased production of CCL and CXCL chemokines and a major influx of dendritic cells (DCs) and neutrophils, with a higher proportion of DCs showing an activated phenotype (high CD80/CD86 expression). Cytokine levels measured in bronchoalveolar-lavage samples showed a small increase in the production of tumor necrosis factor alpha and similar levels of the other cytokines measured (interleukin 10 [IL-10], IL-12, and gamma interferon [IFN-gamma]) in immunized mice compared with control mice. However, the recall response of primed animals after antigenic challenge induced increased expression of IL-12 and IFN-gamma mRNAs in lung homogenates. Overall, all these effects were not due to the lipopolysaccharide content in the bacterial extract. Furthermore, we found that three i.n. doses administered 2 to 3 weeks apart were enough to elicit long-lasting specific serum immunoglobulin G (IgG) and secretory IgA antibody responses. Assessment of IgG subclasses showed a balanced pattern of IgG1-IgG2a responses. The serum total IgE concentrations were also elevated in immunized mice 2 weeks after the third dose, but they significantly decreased soon afterwards. Our results suggest that simple formulations of bacterial extracts administered i.n. are highly immunogenic, eliciting local and systemic immune responses, and may serve as the basis for cost-effective immunotherapies for the prevention and treatment of respiratory infections.
Figures






Similar articles
-
Intranasal vaccination with a secreted chlamydial protein enhances resolution of genital Chlamydia muridarum infection, protects against oviduct pathology, and is highly dependent upon endogenous gamma interferon production.Infect Immun. 2007 Feb;75(2):666-76. doi: 10.1128/IAI.01280-06. Epub 2006 Nov 21. Infect Immun. 2007. PMID: 17118987 Free PMC article.
-
Inactivated Francisella tularensis live vaccine strain protects against respiratory tularemia by intranasal vaccination in an immunoglobulin A-dependent fashion.Infect Immun. 2007 May;75(5):2152-62. doi: 10.1128/IAI.01606-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296747 Free PMC article.
-
The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells.J Immunol. 2003 May 15;170(10):5165-75. doi: 10.4049/jimmunol.170.10.5165. J Immunol. 2003. PMID: 12734364
-
Immunization with Single Oral Dose of Alginate-Encapsulated BCG Elicits Effective and Long-Lasting Mucosal Immune Responses.Scand J Immunol. 2015 Dec;82(6):489-97. doi: 10.1111/sji.12351. Scand J Immunol. 2015. PMID: 26286252
-
Mucosal immunization with purified flagellin from Salmonella induces systemic and mucosal immune responses in C3H/HeJ mice.Vaccine. 2004 Sep 9;22(27-28):3797-808. doi: 10.1016/j.vaccine.2003.12.035. Vaccine. 2004. PMID: 15315861
Cited by
-
Promising Immunomodulatory Effects of Bacterial Lysates in Allergic Diseases.Front Immunol. 2022 Jun 22;13:907149. doi: 10.3389/fimmu.2022.907149. eCollection 2022. Front Immunol. 2022. PMID: 35812388 Free PMC article. Review.
-
Polyvalent Bacterial Lysate Protects Against Pneumonia Independently of Neutrophils, IL-17A or Caspase-1 Activation.Front Immunol. 2021 Apr 26;12:562244. doi: 10.3389/fimmu.2021.562244. eCollection 2021. Front Immunol. 2021. PMID: 33981296 Free PMC article.
-
Immunostimulants versus placebo for preventing exacerbations in adults with chronic bronchitis or chronic obstructive pulmonary disease.Cochrane Database Syst Rev. 2022 Nov 14;11(11):CD013343. doi: 10.1002/14651858.CD013343.pub2. Cochrane Database Syst Rev. 2022. PMID: 36373977 Free PMC article.
-
Immunomodulatory Effects of Pulmonarom®: In Vitro Induction of TLR and Cytokine Expression in Human Dendritic Cells.Pharmaceuticals (Basel). 2025 Jun 13;18(6):885. doi: 10.3390/ph18060885. Pharmaceuticals (Basel). 2025. PMID: 40573281 Free PMC article.
-
Mucosal administration of flagellin protects mice from Streptococcus pneumoniae lung infection.Infect Immun. 2010 Oct;78(10):4226-33. doi: 10.1128/IAI.00224-10. Epub 2010 Jul 19. Infect Immun. 2010. PMID: 20643849 Free PMC article.
References
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Bartlett, J. G., P. O'Keefe, F. P. Tally, T. J. Louie, and S. L. Gorbach. 1986. Bacteriology of hospital-acquired pneumonia. Arch. Intern. Med. 146:868-871. - PubMed
-
- Bellanti, J. A., and B. J. Zeligs. 1994. Current concepts of immune interventions in children with respiratory diseases. Respiration 61(Suppl. 1):3-7. - PubMed
-
- Berman, S. 1995. Otitis media in children. N. Engl. J. Med. 332:1560-1565. - PubMed
-
- Berstad, A. K., F. Oftung, G. E. Korsvold, I. L. Haugen, L. O. Froholm, J. Holst, and B. Haneberg. 2000. Induction of antigen-specific T cell responses in human volunteers after intranasal immunization with a whole-cell pertussis vaccine. Vaccine 18:2323-2330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

