Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C
- PMID: 15102785
- PMCID: PMC387891
- DOI: 10.1128/IAI.72.5.2753-2761.2004
Mucosal and cellular immune responses elicited by recombinant Lactococcus lactis strains expressing tetanus toxin fragment C
Abstract
The mucosal and cellular responses of mice were studied, following mucosal-route administration of recombinant Lactococcus lactis expressing tetanus toxin fragment C (TTFC), which is a known immunogen protective against tetanus. A TTFC-specific T-cell response with a mixed profile of T-helper (Th) subset-associated cytokines was elicited in the intestine, with a Th2 bias characteristic of a mucosal response. These results correlated with the humoral response, where equivalent titers of anti-TTFC immunoglobulin G1 (IgG1) and IgG2a in serum were accompanied by an elevated IgA-specific response at more than one mucosal site. The route of vaccination had an important role in determining the immune response phenotype, as evidenced by the fact that an IgG1-biased subclass profile was obtained when lactococci were administered parenterally. Stimulation of splenic or mesenteric lymph node cells with lactococci resulted in their proliferation and the secretion of gamma interferon via antigen-specific and innate immune mechanisms. The data therefore provide further evidence of the potential of recombinant lactococcal vaccines for inducing systemic and mucosal immune responses.
Figures


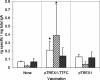

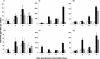
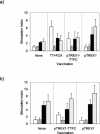
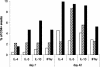
Similar articles
-
Oral vaccination of mice against tetanus with recombinant Lactococcus lactis.Nat Biotechnol. 1997 Jul;15(7):653-7. doi: 10.1038/nbt0797-653. Nat Biotechnol. 1997. PMID: 9219268
-
Progress in the development of Lactococcus lactis as a recombinant mucosal vaccine delivery system.Folia Microbiol (Praha). 1995;40(3):225-30. doi: 10.1007/BF02814197. Folia Microbiol (Praha). 1995. PMID: 8919927 Review.
-
Protection against tetanus toxin after intragastric administration of two recombinant lactic acid bacteria: impact of strain viability and in vivo persistence.Vaccine. 2002 Sep 10;20(27-28):3304-9. doi: 10.1016/s0264-410x(02)00301-8. Vaccine. 2002. PMID: 12213400
-
Enhanced mucosal delivery of antigen with cell wall mutants of lactic acid bacteria.Infect Immun. 2004 May;72(5):2731-7. doi: 10.1128/IAI.72.5.2731-2737.2004. Infect Immun. 2004. PMID: 15102782 Free PMC article.
-
Lactic acid bacteria as live vaccines.Curr Issues Mol Biol. 2000 Jan;2(1):17-25. Curr Issues Mol Biol. 2000. PMID: 11464916 Review.
Cited by
-
Oral administration of Lactococcus lactis expressing Helicobacter pylori Cag7-ct383 protein induces systemic anti-Cag7 immune response in mice.FEMS Immunol Med Microbiol. 2009 Dec;57(3):257-68. doi: 10.1111/j.1574-695X.2009.00605.x. Epub 2009 Sep 1. FEMS Immunol Med Microbiol. 2009. PMID: 19807786 Free PMC article.
-
Improving M cell mediated transport across mucosal barriers: do certain bacteria hold the keys?Immunology. 2004 Sep;113(1):15-22. doi: 10.1111/j.1365-2567.2004.01964.x. Immunology. 2004. PMID: 15312131 Free PMC article. Review.
-
Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells.Appl Environ Microbiol. 2006 Nov;72(11):7091-7. doi: 10.1128/AEM.01325-06. Epub 2006 Sep 8. Appl Environ Microbiol. 2006. PMID: 16963550 Free PMC article.
-
A new plasmid vector for DNA delivery using lactococci.Genet Vaccines Ther. 2009 Feb 10;7:4. doi: 10.1186/1479-0556-7-4. Genet Vaccines Ther. 2009. PMID: 19208231 Free PMC article.
-
Is Lactococcus lactis a Suitable Candidate for Use as a Vaccine Delivery System Against Helicobacter pylori?Curr Microbiol. 2024 Dec 6;82(1):30. doi: 10.1007/s00284-024-03994-1. Curr Microbiol. 2024. PMID: 39643816 Review.
References
-
- Bermudez-Humaran, L. G., P. Langella, N. G. Cortes-Perez, A. Gruss, R. S. Tamez-Guerra, S. C. Oliveira, O. Saucedo-Cardenas, R. Montes de Oca-Luna, and Y. Le Loir. 2003. Intranasal immunization with recombinant Lactococcus lactis secreting murine interleukin-12 enhances antigen-specific Th1 cytokine production. Infect. Immun. 71:1887-1896. - PMC - PubMed
-
- Brandtzaeg, P. 2003. Role of secretory antibodies in the defence against infections. Int. J. Med. Microbiol. 293:3-15. - PubMed
-
- Chamberlain, L. M., J. M. Wells, K. Robinson, K. M. Schofield, and R. W. F. Le Page. 1997. Mucosal immunization with recombinant Lactococcus lactis, p. 83-106. In G. Pozzi and J. M. Wells (ed.), Gram-positive bacteria as vaccine vehicles for mucosal immunization. Landes Bioscience, Austin, Tex.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

