Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesin
- PMID: 15102789
- PMCID: PMC387856
- DOI: 10.1128/IAI.72.5.2791-2802.2004
Proteolytic processing of the Mycoplasma hyopneumoniae cilium adhesin
Abstract
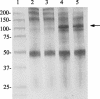
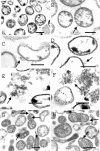
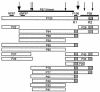
Mycoplasma hyopneumoniae is an economically significant swine pathogen that colonizes the respiratory ciliated epithelial cells. Cilium adherence is mediated by P97, a surface protein containing a repeating element (R1) that is responsible for binding. Here, we show that the cilium adhesin is proteolytically processed on the surface. Proteomic analysis of strain J proteins identified cleavage products of 22, 28, 66, and 94 kDa. N-terminal sequencing showed that the 66- and 94-kDa proteins possessed identical N termini and that the 66-kDa variant was generated by cleavage of the 28-kDa product from the C terminus. The 22-kDa product represented the N-terminal 195 amino acids of the cilium adhesin preprotein, confirming that the hydrophobic leader signal sequence is not cleaved during translocation across the membrane. Comparative studies of M. hyopneumoniae strain 232 showed that the major cleavage products of the cilium adhesin are similar, although P22 and P28 appear to be processed further in strain 232. Immunoblotting studies using antisera raised against peptide sequences within P22 and P66/P94 indicate that processing is complex, with cleavage occurring at different frequencies within multiple sites, and is strain specific. Immunogold electron microscopy showed that fragments containing the cilium-binding site remained associated with the cell surface whereas cleavage products not containing the R1 element were located elsewhere. Not all secreted proteins undergo multiple cleavage, however, as evidenced by the analysis of the P102 gene product. The ability of M. hyopneumoniae to selectively cleave its secreted proteins provides this pathogen with a remarkable capacity to alter its surface architecture.
Figures




References
-
- Cordwell, S. J., D. J. Basseal, B. Bjellqvist, D. C. Shaw, and I. Humphery-Smith. 1997. Characterisation of basic proteins from Spiroplasma melliferum using novel immobilised pH gradients. Electrophoresis 18:1393-1398. - PubMed
-
- Coutte, L., E. Willery, R. Antoine, H. Drobecq, C. Locht, and F. Jacob-Dubuisson. 2003. Surface anchoring of bacterial subtilisin important for maturation function. Mol. Microbiol. 49:529-539. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

