Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses
- PMID: 15102791
- PMCID: PMC387897
- DOI: 10.1128/IAI.72.5.2810-2816.2004
Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses
Abstract
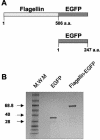
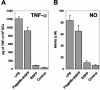
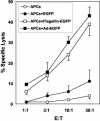
Vaccination is the most efficient prophylaxis against a variety of infectious diseases. New vaccination strategies rely on the incorporation of effective adjuvants, which stimulate the innate immune response and, in turn, activate the adaptive immune response. It is well established that flagellin induces inflammatory responses through the activation of antigen-presenting cells (APCs). In order to evaluate whether flagellin can serve as a carrier for the development of adjuvants or vaccines, we prepared a flagellin-enhanced green fluorescent protein (EGFP) fusion protein. Our results demonstrate that a flagellin-EGFP fusion protein is capable of stimulating APCs, resulting in the maturation of these cells and secretion of proinflammatory cytokines. Furthermore, APCs pulsed with the flagellin-EGFP fusion protein effectively process and present EGFP antigens. More importantly, animals immunized with the flagellin-EGFP fusion protein developed specific anti-EGFP T-cell responses. In contrast, recombinant EGFP was not able to stimulate APCs, nor did it induce a T-cell response. Thus, recombinant-flagellin fusion proteins may be suitable carriers as adjuvants or vaccines for the development of new vaccination strategies to induce and boost immune responses against infectious diseases and cancer.
Figures




Similar articles
-
Enhanced antigen processing of flagellin fusion proteins promotes the antigen-specific CD8+ T cell response independently of TLR5 and MyD88.J Immunol. 2011 Jun 1;186(11):6255-62. doi: 10.4049/jimmunol.1001855. Epub 2011 Apr 22. J Immunol. 2011. PMID: 21515787 Free PMC article.
-
Vaccination with recombinant fusion proteins incorporating Toll-like receptor ligands induces rapid cellular and humoral immunity.Vaccine. 2007 Jan 8;25(4):763-75. doi: 10.1016/j.vaccine.2006.08.013. Epub 2006 Aug 22. Vaccine. 2007. PMID: 16968658
-
A fusion protein of flagellin and ovalbumin suppresses the TH2 response and prevents murine intestinal allergy.J Allergy Clin Immunol. 2011 Dec;128(6):1340-1348.e12. doi: 10.1016/j.jaci.2011.07.036. Epub 2011 Aug 26. J Allergy Clin Immunol. 2011. PMID: 21872305
-
Biotechnology approaches to produce potent, self-adjuvanting antigen-adjuvant fusion protein subunit vaccines.Biotechnol Adv. 2017 May-Jun;35(3):375-389. doi: 10.1016/j.biotechadv.2017.03.005. Epub 2017 Mar 11. Biotechnol Adv. 2017. PMID: 28288861 Review.
-
Utilizing bacterial flagellins against infectious diseases and cancers.Antonie Van Leeuwenhoek. 2014 Feb;105(2):275-88. doi: 10.1007/s10482-013-0075-2. Epub 2013 Nov 26. Antonie Van Leeuwenhoek. 2014. PMID: 24276957 Review.
Cited by
-
The adjuvant activity of alphavirus replicons is enhanced by incorporating the microbial molecule flagellin into the replicon.PLoS One. 2013 Jun 13;8(6):e65964. doi: 10.1371/journal.pone.0065964. Print 2013. PLoS One. 2013. PMID: 23785460 Free PMC article.
-
A recombinant flagellin-poxvirus fusion protein vaccine elicits complement-dependent protection against respiratory challenge with vaccinia virus in mice.Viral Immunol. 2010 Apr;23(2):201-10. doi: 10.1089/vim.2009.0107. Viral Immunol. 2010. PMID: 20374000 Free PMC article.
-
FimH can directly activate human and murine natural killer cells via TLR4.Mol Ther. 2010 Jul;18(7):1379-88. doi: 10.1038/mt.2010.75. Epub 2010 May 4. Mol Ther. 2010. PMID: 20442710 Free PMC article.
-
A fusion protein vaccine containing OprF epitope 8, OprI, and type A and B flagellins promotes enhanced clearance of nonmucoid Pseudomonas aeruginosa.Infect Immun. 2009 Jun;77(6):2356-66. doi: 10.1128/IAI.00054-09. Epub 2009 Apr 6. Infect Immun. 2009. PMID: 19349426 Free PMC article.
-
Bacterial flagellin-a potent immunomodulatory agent.Exp Mol Med. 2017 Sep 1;49(9):e373. doi: 10.1038/emm.2017.172. Exp Mol Med. 2017. PMID: 28860663 Free PMC article. Review.
References
-
- Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. - PubMed
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Barton, G. M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380-383. - PubMed
-
- Ben-Yedidia, T., and R. Arnon. 1998. Effect of pre-existing carrier immunity on the efficacy of synthetic influenza vaccine. Immunol. Lett. 64:9-15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

