Bacterial and host factors involved in the major histocompatibility complex class Ib-restricted presentation of Salmonella Hsp 60: novel pathway
- PMID: 15102795
- PMCID: PMC387849
- DOI: 10.1128/IAI.72.5.2843-2849.2004
Bacterial and host factors involved in the major histocompatibility complex class Ib-restricted presentation of Salmonella Hsp 60: novel pathway
Abstract
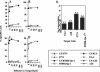
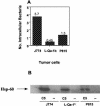
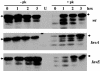
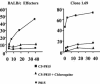
Previously, a peptide epitope derived from the Hsp 60 molecule of Salmonella that is presented by the major histocompatibility complex (MHC) class Ib molecule Qa-1 to CD8(+) cytotoxic T cells (CTLs) was described. In the present study we investigated the Salmonella-induced processing and presentation pathway for generating this Qa-1-restricted epitope. Live bacteria and, to a lesser extent, opsonized heat-killed bacteria are able to sensitize target cells for lysis by Salmonella-specific CTL. In contrast, heat-killed bacteria cannot sensitize target cells. Presentation of the Hsp 60 epitope appears independent of bacterial internalization, because cytochalasin D does not affect presentation. Moreover, Salmonella strains defective in the InvA or InvE operon, two critical components of the type III secretion pathway, are as efficient as wild-type Salmonella enterica serovar Typhimurium in sensitizing infected targets to lysis. Collectively, these results suggest the existence of a novel antigen-processing pathway in which exogenous antigens gain access to the cytosolic MHC class I processing machinery. Considering the abundant nature of bacterial Hsp 60 and the upregulation of this protein after Salmonella infection of eukaryotic cells, this mode of antigen presentation may be particularly relevant to understanding the host defense mechanisms against gram-negative bacteria.
Figures



Similar articles
-
H2-M3 major histocompatibility complex class Ib-restricted CD8 T cells induced by Salmonella enterica serovar Typhimurium infection recognize proteins released by Salmonella serovar Typhimurium.Infect Immun. 2005 Dec;73(12):8002-8. doi: 10.1128/IAI.73.12.8002-8008.2005. Infect Immun. 2005. PMID: 16299293 Free PMC article.
-
T cell responses to Gram-negative intracellular bacterial pathogens: a role for CD8+ T cells in immunity to Salmonella infection and the involvement of MHC class Ib molecules.J Immunol. 1999 May 1;162(9):5398-406. J Immunol. 1999. PMID: 10228017
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
-
The involvement of class Ib molecules in the host response to infection with Salmonella and its relevance to autoimmunity.Microbes Infect. 2001 Nov-Dec;3(14-15):1249-59. doi: 10.1016/s1286-4579(01)01485-x. Microbes Infect. 2001. PMID: 11755413 Review.
-
Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine.J Immunol. 2004 Nov 1;173(9):5852-62. doi: 10.4049/jimmunol.173.9.5852. J Immunol. 2004. PMID: 15494539
Cited by
-
Overlapping Peptide Library to Map Qa-1 Epitopes in a Protein.J Vis Exp. 2017 Dec 20;(130):56401. doi: 10.3791/56401. J Vis Exp. 2017. PMID: 29286392 Free PMC article.
-
Heat-shock proteins: inflammatory versus regulatory attributes.Cell Stress Chaperones. 2008 Summer;13(2):119-25. doi: 10.1007/s12192-008-0018-4. Epub 2008 Feb 28. Cell Stress Chaperones. 2008. PMID: 18758999 Free PMC article. No abstract available.
-
Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells.Eur J Immunol. 2008 Dec;38(12):3411-25. doi: 10.1002/eji.200838432. Eur J Immunol. 2008. PMID: 19009526 Free PMC article.
-
Evaluation of a Salmonella Strain Lacking the Secondary Messenger C-di-GMP and RpoS as a Live Oral Vaccine.PLoS One. 2016 Aug 18;11(8):e0161216. doi: 10.1371/journal.pone.0161216. eCollection 2016. PLoS One. 2016. PMID: 27537839 Free PMC article.
-
Penetratin tandemly linked to a CTL peptide induces anti-tumour T-cell responses via a cross-presentation pathway.Immunology. 2006 Mar;117(3):329-39. doi: 10.1111/j.1365-2567.2005.02304.x. Immunology. 2006. PMID: 16476052 Free PMC article.
References
-
- Ben-Zvi, A. P., and P. Goloubinoff. 2001. Review: mechanisms of disaggregation and refolding of stable protein aggregates by molecular chaperones. J. Struct. Biol. 135:84-93. - PubMed
-
- Brown, M. L., P. E. Fields, and R. J. Kurlander. 1992. Metabolic requirements for macrophage presentation of Listeria monocytogenes to immune CD8 cells. J. Immunol. 148:555-561. - PubMed
-
- Brumell, J. H., A. J. Perrin, D. L. Goosney, and B. B. Finlay. 2002. Microbial pathogenesis: new niches for salmonella. Curr. Biol. 12:R15-R17. - PubMed
-
- Buchmeier, N. A., and F. Heffron. 1990. Induction of Salmonella stress proteins upon infection of macrophages. Science 248:730-732. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

