Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways
- PMID: 15102798
- PMCID: PMC387916
- DOI: 10.1128/IAI.72.5.2864-2871.2004
Borrelia burgdorferi-induced expression of matrix metalloproteinases from human chondrocytes requires mitogen-activated protein kinase and Janus kinase/signal transducer and activator of transcription signaling pathways
Abstract
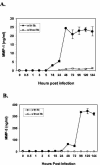
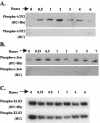
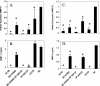
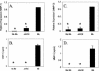
Elevations in matrix metalloproteinase 1 (MMP-1) and MMP-3 have been found in patients with Lyme arthritis and in in vitro models of Lyme arthritis using cartilage explants and chondrocytes. The pathways by which B. burgdorferi, the causative agent of Lyme disease, induces the production of MMP-1 and MMP-3 have not been elucidated. We examined the role of the extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK) and the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways in MMP induction by B. burgdorferi. Infection with B. burgdorferi results in rapid phosphorylation of p38 and JNK within 15 to 30 min. Inhibition of JNK and p38 MAPK significantly reduced B. burgdorferi-induced MMP-1 and MMP-3 expression. Inhibition of ERK1/2 completely inhibited the expression of MMP-3 in human chondrocytes following B. burgdorferi infection but had little effect on the expression of MMP-1. B. burgdorferi infection also induced phosphorylation and nuclear translocation of STAT-3 and STAT-6 in primary human chondrocytes. Expression of MMP-1 and MMP-3 was significantly inhibited by inhibition of JAK3 activity. Induction of MMP-1 and -3 following MAPK and JAK/STAT activation was cycloheximide sensitive, suggesting synthesis of intermediary proteins is required. Inhibition of tumor necrosis factor alpha (TNF-alpha) significantly reduced MMP-1 but not MMP-3 expression from B. burgdorferi-infected cells; inhibition of interleukin-1beta (IL-1beta) had no effect. Treatment of B. burgdorferi-infected cells with JAK and MAPK inhibitors significantly inhibited TNF-alpha induction, consistent with at least a partial role for TNF-alpha in B. burgdorferi-induced MMP-1 expression in chondrocytes.
Figures


References
-
- Beck, G., J. L. Benach, and G. S. Habicht. 1989. Isolation of interleukin 1 from joint fluids of patients with Lyme disease. J. Rheumatol. 16:800-806. - PubMed
-
- Borzì, R. M., I. Mazzetti, L. Cattini, M. Uguccioni, M. Baggiolini, and A. Facchini. 2000. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum. 43:1734-1741. - PubMed
-
- Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

