Mycobacteria inhibit nitric oxide synthase recruitment to phagosomes during macrophage infection
- PMID: 15102799
- PMCID: PMC387846
- DOI: 10.1128/IAI.72.5.2872-2878.2004
Mycobacteria inhibit nitric oxide synthase recruitment to phagosomes during macrophage infection
Abstract
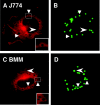
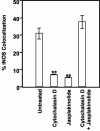
Inducible nitric oxide synthase (iNOS) is a cytoplasmic protein responsible for the generation of nitric oxide (NO. ) in macrophages. In this work, we hypothesized that the intracellular localization of iNOS is significant for effective delivery of NO. to phagosomes containing ingested microorganisms. Using immunofluorescence microscopy and Western blot analysis, iNOS was shown to localize in the vicinity of phagosomes containing latex beads in stimulated macrophages. iNOS also localized to phagosomes containing Escherichia coli. The colocalization of iNOS with ingested latex beads was an actin-dependent process, since treatment with the actin microfilament disrupter cytochalasin D prevented iNOS recruitment to latex bead phagosomes. In contrast to E. coli and inert particle phagosomes, mycobacterial phagosomes did not colocalize with iNOS. This study demonstrates that (i). iNOS can be recruited to phagosomes; (ii). this recruitment is dependent on a functional actin cytoskeleton; (iii). certain microorganisms have the ability to prevent or reduce colocalization with iNOS; and (iv). spatial exclusion of iNOS may play a role in Mycobacterium tuberculosis pathogenesis.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

