Role of macrophages in host resistance to group A streptococci
- PMID: 15102808
- PMCID: PMC387899
- DOI: 10.1128/IAI.72.5.2956-2963.2004
Role of macrophages in host resistance to group A streptococci
Abstract
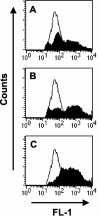
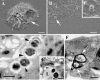
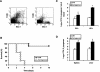
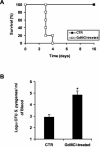
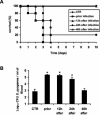
Macrophages provide the first line of defense against invading pathogens. The aim of this study was to determine the role of macrophages during infection with group A streptococci (Streptococcus pyogenes) in mice. Here, we report that resident macrophages can efficiently take up and kill S. pyogenes during in vivo infection, as demonstrated by immunofluorescence and electron microscopy, as well as colony counts. To evaluate the contribution of macrophages to the resolution of experimental infection with S. pyogenes, we compared the susceptibility of BALB/c mice rendered macrophage deficient by treatment with carrageenan with that of intact mice. The results show that depletion of macrophages enhanced the susceptibility of BALB/c mice to S. pyogenes infection, as evidenced by 100% mortality of macrophage-depleted mice compared to 90% survival of nondepleted control animals. The in vivo depletion of macrophages strongly enhanced bacterial loads in the blood and systemic organs. Resistance to S. pyogenes can be restored in macrophage-depleted mice by adoptive transfer of purified macrophages. The in vivo blocking of the macrophage phagocytic function by treatment with gadolinium III chloride also resulted in enhanced susceptibility to S. pyogenes. Interestingly, depletion of macrophages prior to or during the first 24 h of infection decreased survival dramatically; in contrast, no mortality was observed in infected nondepleted animals or mice depleted after 48 h of infection. These results emphasize the important contribution of macrophages to the early control of S. pyogenes infection.
Figures

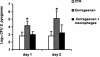
References
-
- Adding, L. C., G. L. Bannenberg, and L. E. Gustafsson. 2001. Basic experimental studies and clinical aspects of gadolinium salts and chelates. Cardiovasc. Drug Rev. 19:41-56. - PubMed
-
- Aderem, A. 2003. Phagocytosis and the inflammatory response. J. Infect. Dis. 187(Suppl. 2):S340-S345. - PubMed
-
- Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. - PubMed
-
- Cone, L. A., D. R. Woodard, P. M. Schlievert, and G. S. Tomory. 1987. Clinical and bacteriologic observations of a toxic shock-like syndrome due to Streptococcus pyogenes. N. Engl. J. Med. 317:146-149. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

