Tick saliva reduces adherence and area of human neutrophils
- PMID: 15102811
- PMCID: PMC387908
- DOI: 10.1128/IAI.72.5.2989-2994.2004
Tick saliva reduces adherence and area of human neutrophils
Abstract
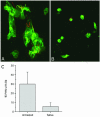
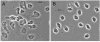
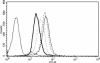
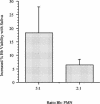
During natural infection with the agent of Lyme disease, Borrelia burgdorferi, spirochetes are delivered with vector saliva, which contains anti-inflammatory and antihemostatic activities. We show here that the saliva of ixodid ticks reduces polymorphonuclear leukocyte (PMN) adhesion via downregulation of beta2-integrins and decreases the efficiency of PMN in the uptake and killing of spirochetes. Inhibition of integrin adhesion and signaling reduces anti-inflammatory functions of PMN. These effects may favor the initial survival of spirochetes in vivo.
Figures
Similar articles
-
Inhibition of neutrophil function by two tick salivary proteins.Infect Immun. 2009 Jun;77(6):2320-9. doi: 10.1128/IAI.01507-08. Epub 2009 Mar 30. Infect Immun. 2009. PMID: 19332533 Free PMC article.
-
Neutrophil extracellular traps entrap and kill Borrelia burgdorferi sensu stricto spirochetes and are not affected by Ixodes ricinus tick saliva.J Immunol. 2012 Dec 1;189(11):5393-401. doi: 10.4049/jimmunol.1103771. Epub 2012 Oct 29. J Immunol. 2012. PMID: 23109724
-
A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent.Cell Host Microbe. 2011 Aug 18;10(2):136-46. doi: 10.1016/j.chom.2011.06.010. Cell Host Microbe. 2011. PMID: 21843870 Free PMC article.
-
Ménage à trois: Borrelia, dendritic cells, and tick saliva interactions.Trends Parasitol. 2014 Feb;30(2):95-103. doi: 10.1016/j.pt.2013.12.003. Epub 2013 Dec 30. Trends Parasitol. 2014. PMID: 24388562 Review.
-
Molecular Interactions During Borrelia burgdorferi Migration from the Vector to the Mammalian Nervous System.Curr Protein Pept Sci. 2020;21(5):517-526. doi: 10.2174/1389203720666191015145714. Curr Protein Pept Sci. 2020. PMID: 31613726 Review.
Cited by
-
Understanding barriers to Borrelia burgdorferi dissemination during infection using massively parallel sequencing.Infect Immun. 2013 Jul;81(7):2347-57. doi: 10.1128/IAI.00266-13. Epub 2013 Apr 22. Infect Immun. 2013. PMID: 23608706 Free PMC article.
-
Borrelia burgdorferi organisms lacking plasmids 25 and 28-1 are internalized by human blood phagocytes at a rate identical to that of the wild-type strain.Infect Immun. 2005 Sep;73(9):5547-53. doi: 10.1128/IAI.73.9.5547-5553.2005. Infect Immun. 2005. PMID: 16113271 Free PMC article.
-
Ticks and Tick-Borne Infections: Complex Ecology, Agents, and Host Interactions.Vet Sci. 2018 Jun 20;5(2):60. doi: 10.3390/vetsci5020060. Vet Sci. 2018. PMID: 29925800 Free PMC article. Review.
-
Borreliae Part 2: Borrelia Relapsing Fever Group and Unclassified Borrelia.Biology (Basel). 2021 Oct 29;10(11):1117. doi: 10.3390/biology10111117. Biology (Basel). 2021. PMID: 34827110 Free PMC article. Review.
-
Purification of a serine protease and evidence for a protein C activator from the saliva of the tick, Ixodes scapularis.Toxicon. 2014 Jan;77:32-9. doi: 10.1016/j.toxicon.2013.10.025. Epub 2013 Oct 31. Toxicon. 2014. PMID: 24184517 Free PMC article.
References
-
- Alekseev, A. N., E. A. Arumova, and I. S. Vasilieva. 1995. Borrelia burgdorferi sensu lato in female cement plug of Ixodes persulcatus ticks (Acari, Ixodiae). Exp. Appl. Acarol. 19:519-522. - PubMed
-
- Barthold, S. W., M. de Souza, E. Fikrig, and D. H. Persing. 1992. Lyme borreliosis in the laboratory mouse, p. 223-242. In S. E. Schutzer (ed.), Lyme disease: molecular and immunologic approaches. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
-
- Coxon, A., P. Rieu, F. J. Barkalow, S. Askari, A. H. Sharpe, U. H. von Andrian, M. A. Arnaout, and T. N. Mayadas. 1996. A novel role for the beta 2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity 5:653-666. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

