Specific Na+ sensors are functionally expressed in a neuronal population of the median preoptic nucleus of the rat
- PMID: 15102913
- PMCID: PMC6729411
- DOI: 10.1523/JNEUROSCI.3720-03.2004
Specific Na+ sensors are functionally expressed in a neuronal population of the median preoptic nucleus of the rat
Abstract
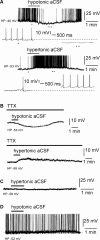
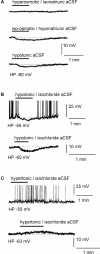
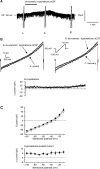
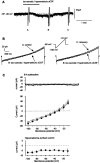
Whole-cell patch-clamp recordings were performed on acute brain slices of male rats to investigate the ability of the neurons of the median preoptic nucleus (MnPO) to detect fluctuation in extracellular osmolarity and sodium concentration ([Na+]out). Local application of hypotonic and hypertonic artificial CSF hyperpolarized and depolarized the neurons, respectively. Similar responses obtained under synaptic isolation (0.5 microM TTX) highlighted the intrinsic ability of the MnPO neurons to detect changes in extracellular osmolarity and [Na+]out. Manipulating extracellular osmolarity, [Na+]out, and [Cl-]out showed in an independent manner that the MnPO neurons responded to a change in [Na+]out exclusively. The specific Na+ response was voltage insensitive and depended on the driving force for Na+ ions, indicating that a sustained background Na+ permeability controlled the membrane potential of the MnPO neurons. This specific response was not reduced by Gd3+, amiloride, or benzamil, ruling out the participation of mechanosensitive cationic channels, specific epithelial Na+ channels, and Phe-Met-Arg-Phe-gated Na+ channels, respectively. Combination of in situ hybridization, using a riboprobe directed against the atypical Na+ channel (Na(X)), and immunohistochemistry, using an antibody against neuron-specific nuclei protein, revealed that a substantial population of MnPO neurons expressed the Na(X) channel, which was characterized recently as a concentration-sensitive Na+ channel. This study shows that a neuronal population of the MnPO acts as functional Na+ sensors and that the Na(X) channel might represent the molecular basis for the extracellular sodium level sensing in these neurons.
Figures



Similar articles
-
Neuronal sodium leak channel is responsible for the detection of sodium in the rat median preoptic nucleus.J Neurophysiol. 2011 Feb;105(2):650-60. doi: 10.1152/jn.00417.2010. Epub 2010 Nov 17. J Neurophysiol. 2011. PMID: 21084682
-
Regulation of central Na+ detection requires the cooperative action of the NaX channel and α1 Isoform of Na+/K+-ATPase in the Na+-sensor neuronal population.J Neurosci. 2013 Feb 13;33(7):3067-78. doi: 10.1523/JNEUROSCI.4801-12.2013. J Neurosci. 2013. PMID: 23407962 Free PMC article.
-
Intrinsic properties of the sodium sensor neurons in the rat median preoptic nucleus.Am J Physiol Regul Integr Comp Physiol. 2012 Oct 15;303(8):R834-42. doi: 10.1152/ajpregu.00260.2012. Epub 2012 Aug 8. Am J Physiol Regul Integr Comp Physiol. 2012. PMID: 22874426 Free PMC article.
-
Patch-clamp studies on epithelial sodium channels in salivary duct cells.Cell Biochem Biophys. 2002;36(2-3):105-13. doi: 10.1385/cbb:36:2-3:105. Cell Biochem Biophys. 2002. PMID: 12139396 Review.
-
Molecular biology of the amiloride-sensitive epithelial Na+ channel.Exp Physiol. 1996 May;81(3):483-92. doi: 10.1113/expphysiol.1996.sp003951. Exp Physiol. 1996. PMID: 8737081 Review.
Cited by
-
Gq DREADD activation of CaMKIIa MnPO neurons stimulates nitric oxide activity.J Neurophysiol. 2020 Aug 1;124(2):591-609. doi: 10.1152/jn.00239.2020. Epub 2020 Jul 22. J Neurophysiol. 2020. PMID: 32697679 Free PMC article.
-
Putative Mechanism of Salt-Dependent Neurogenic Hypertension: Cell-Autonomous Activation of Organum Vasculosum Laminae Terminalis Neurons by Hypernatremia.Hypertension. 2017 Jan;69(1):20-22. doi: 10.1161/HYPERTENSIONAHA.116.08470. Epub 2016 Nov 28. Hypertension. 2017. PMID: 27895191 Free PMC article. No abstract available.
-
The Emergence of TRP Channels Interactome as a Potential Therapeutic Target in Pancreatic Ductal Adenocarcinoma.Biomedicines. 2023 Apr 13;11(4):1164. doi: 10.3390/biomedicines11041164. Biomedicines. 2023. PMID: 37189782 Free PMC article. Review.
-
Structure-guided unlocking of NaX reveals a non-selective tetrodotoxin-sensitive cation channel.Nat Commun. 2022 Mar 17;13(1):1416. doi: 10.1038/s41467-022-28984-4. Nat Commun. 2022. PMID: 35301303 Free PMC article.
-
Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality.J Neurosci. 2006 Aug 30;26(35):9069-75. doi: 10.1523/JNEUROSCI.0877-06.2006. J Neurosci. 2006. PMID: 16943565 Free PMC article.
References
-
- Akopian AN, Souslova V, Sivilotti L, Wood JN (1997) Structure and distribution of a broadly expressed atypical sodium channel. FEBS Lett 400: 183-187. - PubMed
-
- Aradachi H, Honda K, Negoro H, Kubota T (1996) Median preoptic neurones projecting to the supraoptic nucleus are sensitive to haemodynamic changes as well as to rise in plasma osmolality in rats. J Neuroendocrinol 8: 35-43. - PubMed
-
- Black JA, Yokoyama S, Waxman SG, Oh Y, Zur KB, Sontheimer H, Higashida H, Ransom BR (1994) Sodium channel mRNAs in cultured spinal cord astrocytes: in situ hybridization in identified cell types. Brain Res Mol Brain Res 23: 235-245. - PubMed
-
- Bourque CW, Oliet SHR, Richard D (1994) Osmoreceptors, osmoreception and osmoregulation. Front Neuroendocrinol 15: 231-274. - PubMed
-
- Cox PS, Denton DA, Mouw DR, Tarjan E (1987) Natriuresis induced by localized perfusion within the third cerebral ventricle of sheep. Am J Physiol 252: R1-R6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
