Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission
- PMID: 15102917
- PMCID: PMC6729414
- DOI: 10.1523/JNEUROSCI.5531-03.2004
Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission
Abstract
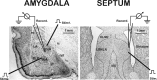
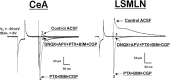
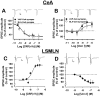
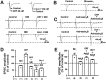
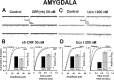
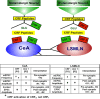
Corticotropin-releasing factor (CRF)-related peptides serve as hormones and neuromodulators of the stress response and play a role in affective disorders. These peptides are known to alter complex behaviors and neuronal properties, but their receptor-mediated effects at CNS synapses are not well described. Here we show that excitatory glutamatergic transmission is modulated by two endogenous CRF-related peptide ligands, corticotropin-releasing factor [CRF rat/human (r/h)] and Urocortin I (Ucn I), within the central nucleus of the amygdala (CeA) and the lateral septum mediolateral nucleus (LSMLN). These limbic nuclei are reciprocally innervated, are involved in stress and affective disorders, and have high densities of the CRF receptors CRF1 and CRF2. Activation of these receptors exerts diametrically opposed actions on glutamatergic transmission in these nuclei. In the CeA, CRF(r/h) depressed excitatory glutamatergic transmission through a CRF1-mediated postsynaptic action, whereas Ucn I facilitated synaptic responses through presynaptic and postsynaptic CRF2-mediated mechanisms. Conversely, in the LSMLN, CRF caused a CRF1-mediated facilitation of glutamatergic transmission via postsynaptic mechanisms, whereas Ucn I depressed EPSCs by postsynaptic and presynaptic CRF2-mediated actions. Furthermore, antagonists of these receptors also affected glutamatergic neurotransmission, indicating that endogenous ligands tonically modulated synoptic activity at these synapses. These data show that CRF receptors in CeA and LSMLN synapses exert and maintain a significant synaptic tone and thereby regulate excitatory glutamatergic transmission. The results also suggest that CRF receptors may provide novel targets in affective disorders and stress.
Figures


Similar articles
-
Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum.J Neurosci. 2005 Jan 19;25(3):577-83. doi: 10.1523/JNEUROSCI.4196-04.2005. J Neurosci. 2005. PMID: 15659593 Free PMC article.
-
CRF modulates glutamate transmission in the central amygdala of naïve and ethanol-dependent rats.Neuropharmacology. 2017 Oct;125:418-428. doi: 10.1016/j.neuropharm.2017.08.009. Epub 2017 Aug 12. Neuropharmacology. 2017. PMID: 28807676 Free PMC article.
-
Corticotropin releasing factor and catecholamines enhance glutamatergic neurotransmission in the lateral subdivision of the central amygdala.Neuropharmacology. 2013 Jul;70:316-23. doi: 10.1016/j.neuropharm.2013.02.014. Epub 2013 Mar 5. Neuropharmacology. 2013. PMID: 23470280 Free PMC article.
-
Urocortins and corticotropin releasing factor type 2 receptors in the hypothalamus and the cardiovascular system.Peptides. 2004 Oct;25(10):1711-21. doi: 10.1016/j.peptides.2004.05.024. Peptides. 2004. PMID: 15476938 Review.
-
Urocortins and the regulation of gastrointestinal motor function and visceral pain.Peptides. 2004 Oct;25(10):1733-44. doi: 10.1016/j.peptides.2004.05.025. Peptides. 2004. PMID: 15476940 Review.
Cited by
-
Inhibitory Control of Basolateral Amygdalar Transmission to the Prefrontal Cortex by Local Corticotrophin Type 2 Receptor.Int J Neuropsychopharmacol. 2020 Feb 1;23(2):108-116. doi: 10.1093/ijnp/pyz065. Int J Neuropsychopharmacol. 2020. PMID: 31800046 Free PMC article.
-
Central CRF neurons are not created equal: phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis.Front Neurosci. 2013 Aug 30;7:156. doi: 10.3389/fnins.2013.00156. eCollection 2013. Front Neurosci. 2013. PMID: 24009552 Free PMC article.
-
A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats.Brain Res. 2007 Jun 25;1155:172-8. doi: 10.1016/j.brainres.2007.04.009. Epub 2007 Apr 10. Brain Res. 2007. PMID: 17512918 Free PMC article.
-
The neuroendocrinology of childhood trauma in personality disorder.Psychoneuroendocrinology. 2012 Jan;37(1):78-86. doi: 10.1016/j.psyneuen.2011.05.006. Epub 2011 Jun 8. Psychoneuroendocrinology. 2012. PMID: 21641725 Free PMC article.
-
Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects.Neuropharmacology. 2016 Mar;102:21-31. doi: 10.1016/j.neuropharm.2015.10.027. Epub 2015 Oct 28. Neuropharmacology. 2016. PMID: 26519902 Free PMC article.
References
-
- Abraham WC, Bear MF (1996) Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126-130. - PubMed
-
- Aggleton JP (1992) The amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. New York: Wiley.
-
- Aldenhoff J, Gruol DL, Rivier J, Vale W, Siggins GR (1983) Corticotropin releasing factor decreases postburst hyperpolarizations and excites hippocampal neurons. Science 221: 875-877. - PubMed
-
- Alheid GF, Heimer L (1988) New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience 27: 1-39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
