The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins
- PMID: 15103330
- PMCID: PMC424389
- DOI: 10.1038/sj.emboj.7600216
The hydrogenase-like Nar1p is essential for maturation of cytosolic and nuclear iron-sulphur proteins
Abstract
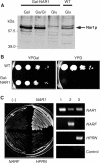
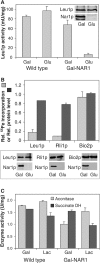
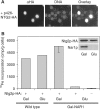
The genome of the yeast Saccharomyces cerevisiae encodes the essential protein Nar1p that is conserved in virtually all eukaryotes and exhibits striking sequence similarity to bacterial iron-only hydrogenases. A human homologue of Nar1p was shown previously to bind prenylated prelamin A in the nucleus. However, yeast neither exhibits hydrogenase activity nor contains nuclear lamins. Here, we demonstrate that Nar1p is predominantly located in the cytosol and contains two adjacent iron-sulphur (Fe/S) clusters. Assembly of its Fe/S clusters crucially depends on components of the mitochondrial Fe/S cluster biosynthesis apparatus such as the cysteine desulphurase Nfs1p, the ferredoxin Yah1p and the ABC transporter Atm1p. Using functional studies in vivo, we show that Nar1p is required for maturation of cytosolic and nuclear, but not of mitochondrial, Fe/S proteins. Nar1p-depleted cells do not accumulate iron in mitochondria, distinguishing these cells from mutants in components of the mitochondrial Fe/S cluster biosynthesis apparatus. In conclusion, Nar1p represents a crucial, novel component of the emerging cytosolic Fe/S protein assembly machinery that catalyses an essential and ancient process in eukaryotes.
Figures





References
-
- Adams MW (1987) The mechanisms of H2 activation and CO binding by hydrogenase I and hydrogenase II of Clostridium pasteurianum. J Biol Chem 262: 15054–15061 - PubMed
-
- Alseth I, Eide L, Pirovano M, Rognes T, Seeberg E, Bjoras M (1999) The Saccharomyces cerevisiae homologues of endonuclease III from Escherichia coli, Ntg1 and Ntg2, are both required for efficient repair of spontaneous and induced oxidative DNA damage in yeast. Mol Cell Biol 19: 3779–3787 - PMC - PubMed
-
- Aris JP, Blobel G (1991) Isolation of yeast nuclei. Methods Enzymol 194: 735–749 - PubMed
-
- Barton RM, Worman HJ (1999) Prenylated prelamin A interacts with Narf, a novel nuclear protein. J Biol Chem 274: 30008–30018 - PubMed
-
- Beinert H, Albracht SPJ (1982) New insights, ideas and unanswered questions concerning iron–sulfur clusters in mitochondria. Biochim Biophys Acta 683: 245–277 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

