Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7
- PMID: 15103331
- PMCID: PMC424394
- DOI: 10.1038/sj.emboj.7600217
Phosphorylation-dependent degradation of c-Myc is mediated by the F-box protein Fbw7
Abstract
The F-box protein Skp2 mediates c-Myc ubiquitylation by binding to the MB2 domain. However, the turnover of c-Myc is largely dependent on phosphorylation of threonine-58 and serine-62 in MB1, residues that are often mutated in cancer. We now show that the F-box protein Fbw7 interacts with and thereby destabilizes c-Myc in a manner dependent on phosphorylation of MB1. Whereas wild-type Fbw7 promoted c-Myc turnover in cells, an Fbw7 mutant lacking the F-box domain delayed it. Furthermore, depletion of Fbw7 by RNA interference increased both the abundance and transactivation activity of c-Myc. Accumulation of c-Myc was also apparent in mouse Fbw7-/- embryonic stem cells. These observations suggest that two F-box proteins, Fbw7 and Skp2, differentially regulate c-Myc stability by targeting MB1 and MB2, respectively.
Figures



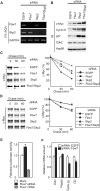
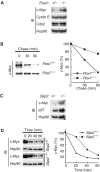
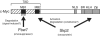
References
-
- Bahram F, von der Lehr N, Cetinkaya C, Larsson LG (2000) c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 95: 2104–2110 - PubMed
-
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A (2003) Role of SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 during S-phase. J Biol Chem 278: 25752–25757 - PubMed
-
- Carrano AC, Eytan E, Hershko A, Pagano M (1999) SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol 1: 193–199 - PubMed
-
- Charrasse S, Carena I, Brondani V, Klempnauer KH, Ferrari S (2000) Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34–SCF(p45Skp2) pathway. Oncogene 19: 2986–2995 - PubMed
-
- Ciechanover A, DiGiuseppe JA, Schwartz AL, Brodeur GM (1991) Degradation of MYCN oncoprotein by the ubiquitin system. Prog Clin Biol Res 366: 37–43 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

