Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering
- PMID: 15103398
- PMCID: PMC389903
- DOI: 10.1251/bpo71
Ribosome formation from subunits studied by stopped-flow and Rayleigh light scattering
Abstract
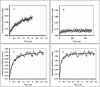
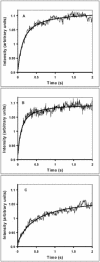
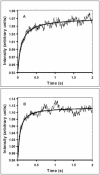
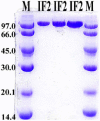
Light scattering and standard stopped-flow techniques were used to monitor rapid association of ribosomal subunits during initiation of eubacterial protein synthesis. The effects of the initiation factors IF1, IF2, IF3 and buffer conditions on subunit association were studied along with the role of GTP in this process. The part of light scattering theory that is essential for kinetic measurements is high-lighted in the main text and a more general treatment of Rayleigh scattering from macromolecules is given in an appendix.
Figures


References
-
- Gualerzi CO, Pon CL. Initiation of mRNA translation in prokaryotes. Biochemistry. 1990;29:5881–5889. - PubMed
LinkOut - more resources
Full Text Sources

