Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo
- PMID: 15105096
- PMCID: PMC400544
- DOI: 10.1128/AAC.48.5.1495-1502.2004
Efficacy of novel hemagglutinin-neuraminidase inhibitors BCX 2798 and BCX 2855 against human parainfluenza viruses in vitro and in vivo
Abstract
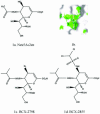
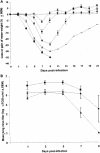
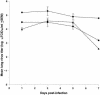
Human parainfluenza viruses are important respiratory tract pathogens, especially of children. However, no vaccines or specific therapies for infections caused by these viruses are currently available. In the present study we characterized the efficacy of the novel parainfluenza virus inhibitors BCX 2798 and BCX 2855, which were designed based on the three-dimensional structure of the hemagglutinin-neuraminidase (HN) protein. The compounds were highly effective in inhibiting hemagglutinin (HA) and neuraminidase (NA) activities and the growth of hPIV-1, hPIV-2, and hPIV-3 in LLC-MK(2) cells. The concentrations required to reduce the activity to 50% of that of a control ranged from 0.1 to 6.0 micro M in HA inhibition assays and from 0.02 to 20 micro M in NA inhibition assays. The concentrations required to inhibit virus replication to 50% of the level of the control ranged from 0.7 to 11.5 micro M. BCX 2798 and BCX 2855 were inactive against influenza virus HA and NA and bacterial NA. In mice infected with a recombinant Sendai virus whose HN gene was replaced with that of hPIV-1 [rSV(hHN)], intranasal administration of BCX 2798 (10 mg/kg per day) and of BCX 2855 (50 mg/kg per day) 4 h before the start of infection resulted in a significant reduction in titers of virus in the lungs and protection from death. Treatment beginning 24 h after the start of infection did not prevent death. Together, our results indicate that BCX 2798 and BCX 2855 are effective inhibitors of parainfluenza virus HN and may limit parainfluenza virus infections in humans.
Figures

References
-
- Appell, L. H., R. M. Kovatch, J. M. Reddecliff, and P. J. Gerone. 1971. Pathogenesis of Sendai virus infection in mice. Am. J. Vet. Res. 32:1835-1841. - PubMed
-
- Bianta, S., C. D. Parker, S. L. Ananth, L. L. Horn, K. Andries, P. Chand, P. L. Kotian, A. Denghani, Y. El-Kattan, T. Lin, T. L. Hutchison, J. A. Montogomery, D. L. Kellog, and Y. S. Babu. 2001. Comparison of the anti-influenza activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162-1167. - PMC - PubMed
-
- Bousse, T., T. Takimoto, W. L. Gorman, T. Takahashi, and A. Portner. 1994. Regions on the hemagglutinin-neuraminidase proteins of human parainfluenza virus type-1 and Sendai virus important for membrane fusion. Virology 204:506-514. - PubMed
-
- Bousse, T., T. Takimoto, T. Matrosovich, and A. Portner. 2001. Two regions of the P protein are required to be active with the L protein for human parainfluenza virus type 1 RNA polymerase activity. Virology 283:306-314. - PubMed
-
- Chanock, R. M., B. R. Murphy, and P. L. Collins. 2001. Parainfluenza viruses, p. 1341-1379. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, Pa.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

