Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141
- PMID: 15105125
- PMCID: PMC400534
- DOI: 10.1128/AAC.48.5.1713-1718.2004
Use of Monte Carlo simulations to select therapeutic doses and provisional breakpoints of BAL9141
Abstract
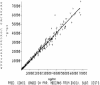
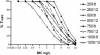
BAL9141, a new antimicrobial agent belonging to the class of parenteral pyrrolidinone-3-ylidenemethyl cephalosporins, is active against most gram-positive microorganisms, including methicillin-resistant variants (methicillin-resistant Staphylococcus aureus [MRSA] and methicillin-resistant Staphylococcus epidermidis [MRSE]), as well as against penicillin-resistant pneumococci (PRP) and many gram-negative microorganisms. BAL9141 is administered as the prodrug BAL5788, which is rapidly converted to BAL9141 by plasma esterases. Pharmacokinetic (PK) data obtained in a previous multiple ascending dose study were used to fit a population PK model to using the NPEM2 program, yielding PK parameter estimates and its covariance matrix for BAL9141. These estimates and matrix were used to perform Monte Carlo simulations (MCSs) and obtain unbiased target attainment rates (TARs) for various time periods during which the concentration remains above the MIC (T(>MIC)). Assuming a T(>MIC) of 40%, TARs of 100% were reached with a dose of 500 mg/liter every 12 h for pathogens with MICs of 2 mg/liter and with a dose of 750 mg/liter every 12 h for pathogens with MICs of 4 mg/liter. Because MICs are </=2 mg/liter for most strains of MRSA, MRSE, and PRP (with some strains showing an MIC of 4 mg/liter), a dosing regimen of 750 mg every 12 h is proposed for clinical studies. The corresponding provisional breakpoint is S (susceptible) </= 4 mg/liter.
Figures


References
-
- Ambrose, P. G., and D. M. Grasela. 2000. The use of Monte Carlo simulation to examine pharmacodynamic variance of drugs: fluoroquinolone pharmacodynamics against Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 38:151-157. - PubMed
-
- Ambrose, P. G., R. C. Owens, Jr., M. J. Garvey, and R. N. Jones. 2002. Pharmacodynamic considerations in the treatment of moderate to severe pseudomonal infections with cefepime. J. Antimicrob. Chemother. 49:445-453. - PubMed
-
- Craig, W. A. 1998. Choosing an antibiotic on the basis of pharmacodynamics. Ear Nose Throat J. 77:7-11. - PubMed
-
- Craig, W. A. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1-10. - PubMed
-
- Daum, R. S., and J. B. Seal. 2001. Evolving antimicrobial chemotherapy for Staphylococcus aureus infections: our backs to the wall. Crit. Care Med. 29:N92-N96. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

