Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening
- PMID: 15105138
- PMCID: PMC400546
- DOI: 10.1128/AAC.48.5.1803-1806.2004
Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening
Abstract
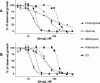
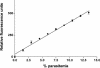
Radioisotopic assays involve expense, multistep protocols, equipment, and radioactivity safety requirements which are problematic in high-throughput drug testing. This study reports an alternative, simple, robust, inexpensive, one-step fluorescence assay for use in antimalarial drug screening. Parasite growth is determined by using SYBR Green I, a dye with marked fluorescence enhancement upon contact with Plasmodium DNA. A side-by-side comparison of this fluorescence assay and a standard radioisotopic method was performed by testing known antimalarial agents against Plasmodium falciparum strain D6. Both assay methods were used to determine the effective concentration of drug that resulted in a 50% reduction in the observed counts (EC(50)) after 48 h of parasite growth in the presence of each drug. The EC(50)s of chloroquine, quinine, mefloquine, artemisinin, and 3,6-bis-epsilon-(N,N-diethylamino)-amyloxyxanthone were similar or identical by both techniques. The results obtained with this new fluorescence assay suggest that it may be an ideal method for high-throughput antimalarial drug screening.
Figures
References
-
- Davis, W., C. Wyatt, M. Hamilton, and W. Goff. 1992. A rapid, reliable method of evaluating growth and viability of intraerythrocytic protozoan hemoparasites using fluorescence flow cytometry. Mem. Inst. Oswaldo Cruz 87:235-239. - PubMed
-
- Guerin, P., P. Olliaro, P. Druilhe, R. Laxminarayan, F. Binka, W. Kilama, N. Ford, and N. White. 2002. Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2:564-573. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

