Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma
- PMID: 15106191
- PMCID: PMC8406989
- DOI: 10.1002/14651858.CD003133.pub2
Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma
Abstract
Background: Anti-leukotriene (AL) agents are being considered as 'add-on' therapy to inhaled corticosteroids (ICS), in chronic asthma.
Objectives: To examine the safety and efficacy of daily AL plus ICS compared to ICS alone, and determine the corticosteroid-sparing effect of AL when added to ICS in chronic asthma.
Search strategy: We searched MEDLINE, EMBASE, CINAHL (until August 2003), reference lists of review articles and trials, contacted international headquarters of AL manufacturers and looked at American Thoracic Society and European Respiratory Society meeting abstracts (1998 to 2003).
Selection criteria: Randomised placebo-controlled trials of asthmatics aged two years and older with at least one month intervention.
Data collection and analysis: Two reviewers assessed quality and extracted data independently. Trials were grouped by asthma control at baseline (symptomatic or well-controlled) and dose of ICS in the control group (same or double).
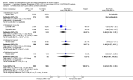
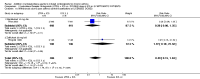
Main results: Of 587 citations, 27 (25 adult and 2 paediatric) trials met inclusion criteria. Sixteen trials were published in full-text and 16 trials reported data in a way that allowed meta-analysis. In symptomatic patients, addition of licensed doses of anti-leukotrienes to ICS resulted in a non-significant reduction in the risk of exacerbations requiring systemic steroids: Relative Risk (RR) 0.64; 95% Confidence Interval (CI) 0.38 to 1.07). A modest improvement group difference in PEF was seen (Weighted Mean Difference (WMD) 7.7 L/min; 95% CI 3.6 to 11.8 L/min) together with decrease in use of rescue short-acting beta2-agonist use (WMD 1 puff/week; 95%CI 0.5 to 2). With only 3 trials comparing the use of licensed doses of anti-leukotrienes with increasing the dose of inhaled glucocorticoids, no firm conclusion can be drawn about the equivalence of both treatment options. In ICS-sparing studies of patients who were well controlled at baseline, addition of anti-leukotrienes produced no overall difference in dose of inhaled glucocorticoids (WMD -21 mcg/d, 95%CI -65, 23 mcg/d), but it was associated with fewer withdrawals due to poor asthma control (RR 0.63, 95% CI 0.42 to 0.95).
Reviewers' conclusions: The addition of licensed doses of anti-leukotrienes to add-on therapy to inhaled glucocorticoids brings modest improvement in lung function. Although addition of anti-leukotrienes to inhaled glucocorticoids appears comparable to increasing the dose of inhaled steroids, the power of the review is insufficient to confirm the equivalence of both treatment options. Addition of anti-leukotrienes is associated with superior asthma control after glucocorticoid tapering; although the glucocorticoid-sparing effect cannot be quantified at present, it appears modest.
Conflict of interest statement
Francine Ducharme has received travel support, research funds and fees for speaking from Zeneca Pharma Inc. producer of zafirlukast and Merck Frosst Inc, producer of montelukast. She has received some travel support for meeting attendance, research grant and consulting fee from Glaxo Wellcome Inc, producer of some inhaled corticosteroids preparation to which anti‐leukotriene agents have been compared. Zachary Schwartz, Giselle Hicks, and Ritz Kakuma: None declared.
Figures
























































































Update of
-
Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma.Cochrane Database Syst Rev. 2002;(1):CD003133. doi: 10.1002/14651858.CD003133. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2004;(2):CD003133. doi: 10.1002/14651858.CD003133.pub2. PMID: 11869653 Updated.
Comment in
-
Review: antileukotriene agents at licensed doses plus inhaled corticosteroids do not reduce asthma exacerbations more than inhaled corticosteroids alone.ACP J Club. 2004 Nov-Dec;141(3):73. ACP J Club. 2004. PMID: 15518457 No abstract available.
-
Evidence-based emergency medicine/systematic review abstract. The role of leukotriene receptor antagonists in asthma care.Ann Emerg Med. 2008 May;51(5):663-5. doi: 10.1016/j.annemergmed.2007.06.492. Ann Emerg Med. 2008. PMID: 18453027 No abstract available.
References
References to studies included in this review
Baba 1999 {published data only}
-
- Baba K, Hattori T, Sakakibara A, Kobayashi T, Takagi K. The usefulness of pranlukast or seratrodast for step‐down of inhaled corticosteroid therapy in adult chronic asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(3 (Part 2 of 2)):A 626.
Bateman 1995 {published and unpublished data}
-
- Bateman ED, Holgate ST, Binks SM, Tarna IP. A multicentre study to assess the steroid‐sparing potential of Accolate (zafirlukast; 20 mg bd). Allergy 1995;50(Suppl 26):320, Abs. P‐0709.
Finn 2000 {published data only}
-
- Finn AF, Bonuccelli CM, Traxler BM, Beatty SE. Zaifirlukast improves asthma control in children treated with and without inhaled corticosteroids. European Respiratory Journal 2000;16(Supplement 31):307.
Green 2002a {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Wardlaw AJ, Pavord ID. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled corticosteroids in subjects with symptomatic asthma despite treatment with low dose inhaled corticosteroids. Thorax 2002;57(Supp III):iii11 (S31).
Hultquist 2000 {unpublished data only}
-
- Hultquist C, Domeij W, Kasak V, Laitinen L, O'Neill S. Oxis turbuhaler (formoterol), accolate (zafirlukast) or placebo as add‐on treatment to pulmicort turbuhaler (budesonide) in asthmatic patients on inhaled steroids. Astra‐Zeneca Report #: SD‐004CR‐0216 2000.
Kanniess 2002 {published and unpublished data}
-
- Kanniess F, Richter K, Janicki S, Schleiss MB, Jorres RA, Magnussen H. Dose reduction of inhaled corticosteroids under concomitant medication with montelukast in patients with asthma. Eur Respir J 2002;20:1080‐1087. - PubMed
Laitinen 1995 {published and unpublished data}
-
- Laitinen LA, Zetterstrom O, Holgate ST, Binks SM, Whitney JG. Effects of Accolate (zafirlukast; 20 mg bd) in permitting reduced therapy with inhaled steroids: a multicenter trial in patients with doses of inhaled steroid optimised between 800 and 2000 mcg per day. Allergy 1995;50 Suppl 26:320, Abs P‐0710.
Laviolette 1999 {published data only}
-
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet J‐C, Peszek I, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(6):1862‐8. - PubMed
Lofdahl 1999 {published and unpublished data}
Nayak 1998 {published and unpublished data}
-
- Nayak AS, Anderson P, Charous BL, Williams K, Simonson S. Equivalence of adding zafirlukast versus double‐dose inhaled corticosteroids in asthmatic patients symptomatic on low‐dose inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;101(1 part 2):S233, Abs 965.
Nishimura 1999 {published data only}
-
- Nishimura K, Hajiro T, Ishihara K, Hasegawa T, Taniguchi H, Katayama S, Ikeda K, Tomii K, Izumi T. Additive effect of pranlukast in combination with inhaled corticosteroid in the treatment of patients with chronic asthma. European Respiratory Journal 1999:P837.
Nsouli 2000 {published data only}
-
- Nsouli SM, McNutt WJ. The addition of montelukast to a low dose inhaled corticosteroid compared with a doubl‐dose of an inhaled corticosteroid in patients with persistent asthma. Annals of Allergy, Asthma & Immunology 2000;84:159.
O'Sullivan 2003 {published data only}
-
- O'Sullivan S, Akveld M, Burke C M, Poulter L W. Effect of the addition of montelukast to inhaled fluticasone propionate on airway inflammation. Am J Respir Crit Care Med 2003;167(5):745‐750. - PubMed
Price 2003 {published data only}
-
- Price D B, Hernandez D, Magyar P, Fiterman J, Beeh K M, James I G, Konstantopoulos S, Rojas R, Noord J A, Pons M, Gilles L, Leff J A. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003;58:0‐6. - PMC - PubMed
Riccioni 2001 {published data only}
-
- Riccioni G, Castronuove M, Benedictis M, Pace‐Palitti V, Gioacchino M, Della Vecchia R, Schiavone C, Sensi S, Guagnano M.T. Zafirlukast versus budesonide on bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology and Pharmacology 2001;14(2):87‐92. - PubMed
Riccioni 2002 {published data only}
-
- Riccioni G, Ballone E, D'Orazio N, Sensi S, Nicola M, Mascio R, Santilli F, Guagnano MT, Della Vecchia R. Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild‐persistent asthma. Int J Immunopathol Pharmacol 2002;15(2):149‐155. - PubMed
Ringdal 1999 {published and unpublished data}
-
- Ringdal N, White M, Harris A. Addition of Zafirlukast (Accolate) compared with a double‐dose of inhaled corticosteroids in patients with reversible airways obstruction symptomatic on inhaled corticosteroids.. American Journal of Respiratory & Critical Care Medicine 1999; Vol. 159, issue 3 part 2:639 Abs.
Shingo 2001 {published and unpublished data}
-
- Shingo S, Zhang J, Noonan N, Reiss TF, Leff JA. A standardized composite clinical score for inhaled corticosteroids taper studies in asthma. Drug Inform J 2002;36:501‐508.
Simons 2001 {published and unpublished data}
-
- Simons FER, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: a randomised, double‐blind, crossover study. Journal of Pediatrics 2001;138(5):694‐8. - PubMed
Tamaoki 1997 {published data only}
-
- Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high‐dose inhaled corticosteroid. American Journal of Respiratory & Critical Care Medicine 1997;155:1235‐40. - PubMed
Tohda 2002 {published and unpublished data}
-
- Tohda Y, Fujimura M, Taniguchi H, Takagi K, Igarashi T, Yasuhara H, Takahashi K, Nakajima S. Leukotriene receptor antagonist, montelukast, can reduce the need for inhaled steroid while maintaining the clinical stability of asthmatic patients. Clin Exp Allergy 2002;32:1180‐1186. - PubMed
Tomari 2001 {published data only}
-
- Tomari S, Shimoda T, Kawano T, Mitsuta K, Obase Y, Fukushima C, Matsuse H, Kohno S. Effects of pranlukast, a cysteinyl leukotriene receptor 1 antagonist, cobined with inhaaled beclomethasone in patients with moderate or severe asthma. Ann Allergy Asthma Immunol 2001;87:156‐161. - PubMed
Tomita 1999 {published data only}
-
- Tomita K, Hashimoto K, Matsumoto S, Nakamoto N, Tokuyasu H, Yamasaki A, et al. Pranlukast allows reduction of inhaled steroid dose without deterioration in lung function in adult asthmatics. Article in Japanese. Arerugi ‐ Japanese Journal of Allergology 1999;48(4):459‐65. - PubMed
Vaquerizo 2003 {published data only}
Virchow 2000 {published and unpublished data}
-
- Virchow JC, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high‐dose inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 2000;162:578‐85. - PubMed
Wada 1999 {published data only}
-
- Wada K, Minoguchi K, Kohno Y, Oda N, Matsuura T, Kawazu K, et al. Effect of a leukotriene receptor antagonist, pranlukast hydrate, on airway inflammation and airway hyperresponsiveness in patients with moderate to severe asthma. Allergology International 2000;49(1):63‐8.
Yildirim 2001 {published data only}
-
- Yildirim Z, Ozlu T, Bulbul Y. Montelukast and budesonide vs double dose budesonide in moderate asthma. European Respiratory Journal 2001;18(Supp 33):261s.
References to studies excluded from this review
Altman 1998 {published data only}
-
- Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, et al. A placebo‐controlled, dose‐ranging study of montelukast, a cysteinyl leukotriene‐receptor antagonist. Journal of Allergy & Clinical Immunology 1998;102:50‐6. - PubMed
Barnes 1996 {published data only}
-
- Barnes NC, Black B, Syrett N, Cohn J. Reduction of exacerbations of asthma in multi‐national clinical trials with zafirlukast (Accolate). Allergy 1996;51(Suppl 30):84.
Barnes 1997 {published data only}
Basyigit 2001 {published data only}
-
- Basyigit IE, Yildiz F, Özkara SK, Boyaci H, Ilgazli A, Özkarakas O. The effects of inhaled steroids, leukotriene receptor antagonists and theophylline on induced sputum ECP levels mild persistent asthma. European Respiratory Journal 2001;18(Supp 33):261s.
Baumgartner 1999 {unpublished data only}
-
- Baumgartner RA, Polis A, Angner R, Bird S, Reiss TF. Comparison between montelukast and inhaled beclomethasone therapy in chronic asthma: a double‐blind, placebo‐controlled, parallel study in asthmatic patients. Merck Research Laboratories 1999.
Becker 2000 {published data only}
-
- Becker A. Leukotriene receptor antagonists: efficacy and safety in children with asthma. Pediatric Pulmonology 2000;30(2):183‐6. - PubMed
Bisgaard 1999 {published data only}
-
- Bisgaard H, Loland L, Anhoj J. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. American Journal of Respiratory & Critical Care Medicine 1999;160:1227‐31. - PubMed
Bisgaard 2000 {published data only}
-
- Bisgaard H, Nielsen KG. Bronchoprotection with a leukotriene receptor antagonist in asthmatic preschool children. American Journal of Respiratory & Critical Care Medicine 2000;162(1):187‐90. - PubMed
Bjermer 2000 {published data only}
-
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening A, Haahtela T, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double‐blind comparative study. Respiratory Medicine 2000;94(6):612‐21. - PubMed
Bjermer 2002 {published data only}
-
- Bjermer L, Greening A, Haahtela T, Bousquet J, Holgate ST, Picado C, Bisgaard H, Fabbri L, Menten J, Lef JA, IMPACT study group. Addition of montelukast or salmeterol to fluticasone in patients with uncontrolled asthma: results of the IMPACT trial.. Chest 2002:434.
Brabson 2002 {published data only}
-
- Brabson J H, Clifford D, Kerwin E, Raphael G, Pepsin P J, Edwards L D, Srebro S, Rickard K. Efficacy and safety of low‐dose fluticasone propionate compared with zafirlukast in patients with persistent asthma. American Journal of Medicine 2002;113(1):15‐21. - PubMed
Brannan 2001 {published data only}
-
- Brannan JD, Anderson SD, Gomes K, King GG, Chan HK, Seale JP. Fenofenadine decreases sensitivity to and montelukast improves recovery from inhaled manitol. American Journal of Respiratory & Critical Care Medicine 2001;163(6):1420‐5. - PubMed
Brocks 1996 {published data only}
-
- Brocks DR, Upward JW, Georgiou P, Stelman G, Doyle E, Allen E, et al. The single and multiple dose pharmacokinetics of pranlukast in healthy volunteers. European Journal of Clinical Pharmacology 1996;51:303‐8. - PubMed
Bronsky 1997 {published data only}
-
- Bronsky E, Grossman J, Nathan RA, Jong B. Pranlukast (Ultair) reduces symptoms of seasonal allergic rhinitis: results of the first US double‐blind, placebo controlled trial in 484 patients. SmithKline Beecham Pharmaceuticals 1997.
Bruce 2002 {published data only}
-
- Bruce C, Palmqvist M, Sjöstrand M, Aronsson B, Arvidsson P, Lotvall J. Greater attenuation of the late asthmatic reaction by fluticasone propionate compared to montelukast. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215.
Busse 1999 {published data only}
-
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy & Clinical Immunology 1999;103:1075‐80. - PubMed
Cakmak 2000 {published data only}
-
- Cakmak G, Demir T, Aydemir A, Serdaroglu E, Erginoz E, Donma O, Gemicioglu B. The effect of adding zafirlukast to budesonide treatment on total antoxydant capacity. European Respiratory Journal 2000;16(Supplement 31):457s.
Calhoun 1997 {published data only}
-
- Calhoun WJ, Weisberg SC, Faiferman I, Stober PW. Pranlukast (Ultair) is effective in improving asthma: results of a 12‐week, multicenter, dose‐range study. Journal of Allergy & Clinical Immunology 1997;99:S318, Abs 1305.
Calhoun 2001 {published data only}
-
- Calhoun W J, Nelson H S, Nathan R A, Pepsin P J, Kalberg C, Emmett A, Rickard K A, Dorinsky P. Comparison of fluticasone propionate‐salmeterol combination therapy and montelukast in patients who are symptomatic on short‐acting beta(2)‐agonists alone. American Journal of Respiratory & Critical Care Medicine. 2001; 164(5):759‐63. 2001;164(5):759‐763. - PubMed
Camargo 2002 {published data only}
-
- Camargo C A, Smithline H A, Marie‐Pierre M, Green S A, Reiss T F. A Randomized Controlled Trial of Intravenous Montelukast in Acute Asthma. Am J Respir Crit Care Med 2002. - PubMed
Capella 2001 {published data only}
-
- Capella GL, Frigerio E, Altomare G. A randomized trial of leukotriene receptor antagonist montelukast in moderate‐to‐severe atopic dermatitis of adults. European Journal of Dermatology 2001;11(3):209‐13. - PubMed
Chuchalin 2002 {published data only}
-
- Chuchalin A G, Ovcharenko S I, Goriachkina L A, Sidorenko I V, Tsoi A N, Alekseev V G, Bart B Y, Borisova N K, Belousov Y B, Golovko M G, Goriachkina L A, Chereiskaya N K, Daniliak I G, Didkovsky N A, Dobrotina I S, Emelyanov A V, Korovina O V, Mamtsev B N, Sokurenko S I, Valekjanina T G, Zadionchenko V S. The safety and efficacy of formoterol OxisRTurbuhalerR plus budesonide PulmicortRTurbuhaler in mild to moderate asthma: A comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. - PubMed
Clifford 2000 {published data only}
-
- Clifford D, Pepsin P, Srebro S, Edwards L, Rickard K. Fluticasone propionate 88mcg bid is superior to zafilukast 20mg bid in controlling persistent asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A202.
Cloud 1989 {published data only}
-
- Cloud ML, Enas GC, Kemp J, Platts‐Mills T, Altman LC, Townley R, et al. A specific LTD4‐LTE4‐receptor antagonist improves pulmonary function in patients with mild, chronic asthma. American Review of Respiratory Disease 1989;140(5):1336‐9. - PubMed
Currie 2003 {published data only}
-
- Currie G P. Montelukast confers complimentary non‐steroidal anti‐inflammatory activity in asthmatics receiving fluticasone alone and fluticasone/salmeterol combination. J Allergy Clin Immunol 2003;111(2):Abstact 309.
Currie 2003 (B) {published data only}
-
- Currie G P, Lee D K C, Haggart K, Bates C E, Lipworth B J. Effects of montelukast on surrogate inflammatory markers in corticosteroid‐treated patients with asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(9):1232‐1238. - PubMed
Cylly 2003 {published data only}
-
- Cylly A, Kara A, Ozdemir T, Ogus C, Gulkesen K H. Effects of oral montelukast on airway function in acute asthma. Respir Med 2003;97(5):533‐536. - PubMed
Dahlen 2002 {published and unpublished data}
-
- Dahlén SE, Malmstrom K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, et al. Improvement of aspirin‐intolerant asthma by montelukast, a leukotriene antagonist. A randomised, double‐blind, placebo controlled trial. American Journal of Respiratory & Critical Care Medicine 2002;165(1):9‐14. - PubMed
Daikh 2003 {published data only}
-
- Daikh B E, Ryan C K, Schwartz R H. Montelukast reduces peripheral blood eosinophilia but not tissue eosinophilia or symptoms in a patient with eosinophilic gastroenteritis and esophageal stricture. Annals of Allergy, Asthma, & Immunology 2003;90(1):23‐27. - PubMed
Dempsey 1999 {published data only}
-
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. A comparison of once daily topical budesonide (BUD) and oral montelukast (MON) in seasonal allergic rhinitis (SAR) and asthma. European Respiratory Society 1999. - PubMed
Dempsey 2000 {published data only}
-
- Dempsey OJ, Wilson AM, Sims EJ, Mistry C, Lipworth BJ. Additive bronchoprotective and bronchodilator effects with single doses of salmeterol and montelukast in asthmatic patientsreceiving inhaled corticosteroids. Chest 2000;117:950‐3. - PubMed
Dempsey 2000b {published data only}
-
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. Additive anti‐inflammatory effects of montelukast but not salmeterol in asthmatics suboptimally controlled on inhaled steroids [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A198.
Dempsey 2001 {published data only}
-
- Dempsey OJ, Wilson AM, Lipworth BJ. Comparative efficacy and anti‐inflammatory profile of once daily low dose Hfa‐triamcinolone Acetonide (TAA) and Montelukast (ML) in patients with mild persistent atopic asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S316. - PubMed
Dempsey 2002 {published data only}
-
- Dempsey OJ, Fowler SJ, Wilson A, Kennedy G, Lipworth BJ. Effects of adding either a leukotriene receptor antagonist or low‐dose theophylline to a low or medium dose of inhaled corticosteroid in patients with persistent asthma. Chest 2002;122(1):151‐159. - PubMed
Dessanges 1999 {published data only}
-
- Dessanges JF, Prefaut C, Taytard A, Matran R, Naya I, Compagnon A, et al. The effect of zafirlukast on repetitive exercise‐induced bronchoconstriction: The possible role of leukotrienes in exercise‐induced refractoriness. Journal of Allergy & Clinical Immunology 1999;104(6):1155‐61. - PubMed
Diamant 1999 {published data only}
-
- Diamant Z, Grootendorst DC, Veselic‐Charvat M, Timmers MC, Smet M, Leff JA, et al. The effect of montelukast (MK‐0476), a cysteinyl leukotriene receptor antagonist, on allergen‐induced airway responses and sputum cell counts in asthma. Clinical & Experimental Allergy 1999;29(1):42‐51. - PubMed
Dicpinigaitis 2002 {published data only}
-
- Dicpinigaitis P V, Dobkin J B, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. Journal of Asthma 2002;39(4):291‐297. - PubMed
Dockhorn 2000 {published data only}
Eliraz 2001 {published data only}
-
- Eliraz A, Raminez‐Rivera A, Ferranti P, et al. Similar efficacy following four weeks treatment of asthmatics with formoterol 12 mcg bid delivered by two different dry powder inhalers: differences in inhaler handling. International Journal of Clinical Practice 2001;55(3):164‐70. - PubMed
Faul 2002 {published data only}
-
- Faul JL, Canfield JC, Gould MK, Wilson SR, Kischner WG. A randomized, double‐blind, placebo‐controlled, crossover study comparing the effects of inhaled fluticasone propionate (880 Micrograms per day) and montelukast (10 MG per day) on glucose control patients with diabetes and asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217.
Findlay 1992 {published data only}
-
- Findlay SR, Barden JM, Easley CB, Glass M. Effect of the oral leukotriene antagonist, ICI 204,219, on antigen‐induced bronchoconstriction in subjects with asthma. Journal of Allergy & Clinical Immunology 1992;89:1040‐5. - PubMed
Fischer 1995 {published data only}
-
- Fischer AR, McFadden CA, Frantz R, Awni WM, Cohn J, Drazen JM, et al. Effect of chronic 5‐lipoxygenase inhibition on airway hyperresponsiveness in asthmatic subjects. American Journal of Respiratory & Critical Care Medicine 1995;152(4 Part 1):1203‐7. - PubMed
Fischer 1997 {published data only}
Fish 1997 {published data only}
-
- Fish JE, Kemp JP, Lockey RF, Glass M, Hanby L, Bonuccelli CM. Zafirlukast for symptomatic mild‐to‐moderate asthma: a 13‐week multicenter study. Clinical Therapeutics 1997;19(4):675‐90. - PubMed
Flynn 2001 {published data only}
-
- Flynn David. N of 1 Trials of Montelukast (ML) or Singular (Trade Name) in Childhood Asthma. The Royal Free Hampstead NHS Trust.
Fujimura 1993 {published data only}
-
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effect of a leukotriene antagonist, ONO‐1078, on bronchial hyperresponsiveness in patients with asthma. Respiratory Medicine 1993;87:133‐8. - PubMed
Gaddy 1990 {published data only}
-
- Gaddy J, Bush RK, Margolskee D, Williams VC, Busse W. The effects of a leukotriene D4 (LTD4) antagonist (MK‐571) in mild to moderate asthma. Journal of Allergy & Clinical Immunology 1990;85:197, Abs. 216.
Galant 2001 {published data only}
-
- Galant S, Gode‐Sellers S, Kalberg C, Edwards L, Srebro S, Rickard K. Low dose inhaled Fluticasone Propionate provides greater improvement in pulmonary function as compared to Montelukast in patients with persistent asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S106. - PubMed
Geha 2001 {published data only}
-
- Geha RS. Desloratadine: a new, nonsedating, oral antihistamine. Journal of Allergy & Clinical Immunology 2001;107(4):751‐62. - PubMed
Georgiou 1997 {published data only}
-
- Georgiou P, Compton C, Allen A, Hust R, Collie H. Pranlukast (Ultair) has no effect on cardiovascular parameters in healthy male subjects. ATS. 1997:Abs C49.
Ghiro 2002 {published data only}
-
- Ghiro L, Zanconato S, Rampon O, Piovan V, Pasquale M F, Baraldi E. Effect of montelukast added to inhaled corticosteroids on fractional exhaled nitric oxide in asthmatic children. Eur Respir J. 2002;20(3):630‐634. - PubMed
Gold 2001 {published data only}
-
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001;18(Supp 33):262s.
Green 2002b {published data only}
-
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw A J, Pavord I D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002;360(9347):1715‐1721. - PubMed
Grossman 1995 {published data only}
-
- Grossman J, Bronsky E, Busse W, Montanaro A, Southern L, Tinkelman D, et al. A multicenter, double‐blind, placebo‐controlled study to evaluate the safety, tolerability and clinical activity of oral, twice‐daily LTA, Pranlukast (SB 205312) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(1):352, Abs 846.
Grossman 1997 {published data only}
-
- Grossman J, Faiferman I, Dubb JW, Tompson DJ, Busse W, Bronsky E, et al. Results of the first U.S. double‐blind, placebo‐controlled, multicenter clinical study in asthma with pranlukast, a novel leukotriene receptor antagonist. Journal of Asthma 1997;34(4):321‐8. - PubMed
Haahtela 1994 {published data only}
-
- Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. New England Journal of Medicine 1994;331(11):700‐5. - PubMed
Hamilton 1998 {published data only}
-
- Hamilton AL, Faiferman I, Stober P, Watson RM, O'Byrne PM. Pranlukast, a cysteinyl leukotriene receptor antagonist, attenuates allergen‐induced early and late phase bronchoconstriction and airway hyperresponsiveness in asthmatic subjects. Journal of Allergy & Clinical Immunology 1998;102(2):177‐83. - PubMed
Hassall 1998 {published data only}
-
- Hassell SM, Miller C, Harris A. Zafirlukast (Accolate) reduces the need for oral steroid bursts. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Hood 1999 {published data only}
Howland 1994 {published data only}
-
- Howland III W, Segal A, Glass M, Minkwitz MC. 6‐week therapy with the oral leukotriene‐receptor antagonist, ICI 204,219, in the treatment of asthma. Journal of Allergy & Clinical Immunology 1994;93(1):259, Abs. 581.
Hsieh 1996 {published data only}
-
- Hsieh, K‐H. Evaluation of efficacy of traditional chinese medicines in the treatment of childhood bronchial asthma: clinical trial, immunological tests and animal study. Pediatric Allergy & Immunology 1996;7:130‐40. - PubMed
Hughes 1999 {unpublished data only}
-
- Hughes GL, Edelman JM, Turpin JA, Liss C, Weeks K, Schweiger D, et al. Randomized, open‐label pilot study comparing the effects of montelukast sodium tablets, fluticasone aerosol inhaler, and budesonide dry powder inhaler on asthma control in mild asthmatics. Merck Research Laboratories 1999.
Hui 1991 {published data only}
-
- Hui K, Barnes NC. Lung function improvement in asthma with a cysteinyl‐leukotriene receptor antagonist. Lancet 1991;337:1062‐3. - PubMed
Israel 1990 {published data only}
-
- Israel E, Dermarkarkian R, Rosenberg M, Sperling R, Taylor G, Rubin P, et al. The effects of a 5‐lipoxygenase inhibitor on asthma induced by cold, dry air. New England Journal of Medicine 1990;323:1740‐4. - PubMed
Israel 1992 {published data only}
-
- Israel E, Drazen J, Pearlman H, Cohn J, Rubin P. A double‐blind multicenter study of zileuton, a potent 5‐lipoxygenase (5‐LO) inhibitor versus placebo in the treatment of spontaneous asthma in adults. Journal of Allergy & Clinical Immunology 1992;89:236, Abs. 368.
Israel 1993 {published data only}
-
- Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, et al. The effect of inhibition of 5‐lipoxygenase by zileuton in mild‐to‐moderate asthma. Annals of Internal Medicine 1993;119(11):1059‐66. - PubMed
Israel 1996 {published data only}
-
- Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5‐lipoxygenase inhibitor, in patients with asthma. The Journal of the American Medical Association 1996;275(12):931‐6. - PubMed
Israel 2002 {published data only}
-
- Israel E, Chervinsky P S, Friedman B, Bavel J, Skalky C S, Ghannam A F, Bird S R, Edelman J M. Effects of montelukast and beclomethasone on airway function and asthma control. Journal of Allergy & Clinical Immunology 2002;110(6):847‐854. - PubMed
Jayaram 2002 {published data only}
-
- Jayaram L, Pizzichini MMM, Hussack P, Lemiere C, Cartier A, Man SFP, Pizzichini E, Hargreave FE. First line anti‐inflammatory treatment for asthma; inhaled steroid or leukotriene antagonist?. Respirology 2002;7(Supplement):A19.
Johnson 1999 {published data only}
-
- Johnson MC, Srebro S, Edwards L, Bowers B, Rickard K. Physician‐ and patient‐rated assessments correlate well with clinical efficacy measurements in a study comparing fluticasone and zafirlukast. Journal of Allergy and Clinical Immunology 1999;103(1 Part 2):Abs. 882.
Juniper 1995 {published data only}
-
- Juniper EF, Dube L, Swanson LJ, Zileuton Study Group. The effect of zileuton, a 5‐lipoxygenase inhibitor, on asthma quality of life. Asthma. 1995.
Kalberg 1999 {published data only}
-
- Kalberg CJ, Yancey S, Emmett AH, Rickard K. A comparison of salmeterol versus zafirlukast in patients using inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1999;103(1 Part 2):abs 881. - PubMed
Kanniess 2002 (B) {published data only}
-
- Kanniess F, Richter K, Bohme S, Jorres R A, Magnussen H. Montelukast versus fluticasone: effects on lung function, airway responsiveness and inflammation in moderate asthma. Eur Respir J. 2002;20(4):853‐858. - PubMed
Kemp 1995 {published data only}
-
- Kemp JP, Glass M, Minkwitz MC. Onset of action of the leukotriene‐receptor antagonist, zafirlukast (Accolate), in patients with asthma. Journal of Allergy & Clinical Immunology 1995;95(1 Part 2):351, Abs. 844.
Kemp 1997 {published data only}
-
- Kemp JP, et al. Montelukast, a leukotriene receptor antagonist, inhibits exercise induced bronchoconstriction in 6‐14 year old children. Journal of Allergy & Clinical Immunology 1997.
Kemp 1998 {published data only}
-
- Kemp JP, Tinkelman D, Sublett J, Compton C, Georgiou P. Pranlukast (Ultair) pharmacokinetics in children consistent with that of adults. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Kemp 1999 {published data only}
-
- Kemp JP, Minkwitz MC, Bonuccelli CM, Warren MS. Therapeutic effect of zafirlukast as monotherapy in steroid‐naive patients with severe persistent asthma. Chest 1999;115(2):336‐42. - PubMed
Kim 2000 {published data only}
-
- Kim KT, Ginchansky EJ, Srebro S, Pepsin PJ, Edwards L, Stanford RH, et al. Fluticasone propionate versus zafirlukast: effect in patients previously receiving inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 2000;85(5):398‐406. - PubMed
Kips 1991 {published data only}
-
- Kips JC, Joos GF, Lepeleire I, Margolskee DJ, Buntinx A, Pauwels RA, et al. MK‐571, a potent antagonist of leukotriene D4‐induced bronchoconstriction in the human. American Review of Respiratory Disease 1991;144:617‐21. - PubMed
Knorr 1997 {published data only}
-
- Knorr BA, et al. Montelukast improves asthma in children 6‐14 years. American Thoracic Society 1997.
Knorr 1998 {published data only}
-
- Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, et al. Montelukast for chronic asthma in 6‐ to 14‐year‐old children. The Journal of the American Medical Association 1998;279(15):1181‐6. - PubMed
Knorr 1999 {published data only}
-
- Knorr B, Nguycn HH, Seidenberg BC, Reiss TF, Montelukast Pediatric Study Group. Montelukast, a leukotriene receptor antaconist, provides additional clinical benefit in asthmatic children aged 6 to 14 years using inhaled corticosteroids. European Respiratory Society 1999;P363.
Korenblat 1998 {published data only}
-
- Korenblat P, Chervinsky P, Wenzel S, Faiferman I, Bakst A. Pranlukast (Ultair) reduces health care utilization and improves quality of life in adult patients with mild‐to‐moderate asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Kuna 1997 {published data only}
-
- Kuna P, Malmstrom K, Dahlen SE, Nizankowska E, Kowalski M, Stevenson D, et al. Montelukast (MK‐0476), a cysLT1 receptor antagonist, improves asthma control in aspirin‐intolerant asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;155(4):A975.
Kylstra 1998 {published data only}
-
- Kylstra JW, Sweitzer DE, Miller CJ, Bonuccelli CM. Zafirlukast (Accolate) in moderate asthma: patient‐reported outcomes and peripheral eosinophil data from a 13‐week trial. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Laitinen 1997 {published data only}
-
- Laitinen LA, Naya IP, Binks S, Harris A. Comparative efficacy of zafirlukast & low dose steroids in asthmatics on prn beta2‐agonists. European Respiratory Journal 1997;10(Suppl 25):419‐20s, Abs. 2716.
Leff 1998 {published data only}
-
- Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, et al. Montelukast, a leukotriene‐receptor antagonist, for the treatment of mild asthma and exercise‐induced bronchoconstriction. New England Journal of Medicine 1998;339:147‐52. - PubMed
Leigh 2002 {published data only}
-
- Leigh R, Vethanayagam D, Yoshida M, Watson R M, Rerecich T, Inman M D, O'Byrne P M. Effects of montelukast and budesonide on airway responses and airway inflammation in asthma. Am J Respir Crit Care Med 2002;166(9):1212‐1217. - PubMed
Leigh 2002 (B) {published data only}
-
- Leigh R, Vethanayagam D, Yoshida M, Watson RM, Rererich T, Killian K, O'Byrne PM. Effects of montelukast and budesonide alone or in combination on allergen induced early and late asthmatic responses, and post‐allergen airway hyperresponsiveness [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216.
Lipworth 1999 {unpublished data only}
-
- Lipworth BJ. Comparative potency and anti‐inflammatory profile of monotherapy with either montelukast or zafirlukast, patients with mild‐moderate asthma. Academic Publications (Ongoing trial) 1999.
Lipworth 2000 {unpublished data only}
-
- Lipworth BJ. A comparison of anti‐histamine and leukotriene receptor antagonist as steroid sparing agents in patients with atopic asthma.. Ongoing trial 2000.
Liu 1996 {published data only}
-
- Liu MC, Dube LM, Lancaster J, Zileuton Study Group. Acute and chronic effects of a 5‐lipoxygenase inhibitor in asthma: a 6‐month randomized multicenter trial. Journal of Allergy & Clinical Immunology 1996;98:859‐71. - PubMed
Lockey 1995 {published data only}
-
- Lockey RF, Lavins BJ, Snader L. Effects of 13 weeks of treatment with ICI 204,219 (Accolate) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(Part 2):350, Abs. 839.
Malerba 2002 {published data only}
-
- Malerba M, Radaeli A, Ceriani L, Amato M, Tomenzoli D, Nicolai P, Tantucci C, Grassi V. Comparison of oral montelukast and inhaled fluticasone in the treatment of asthma associated with chronic rhinopolyposis: A single‐blind, randomized, pilot study. Current Therapeutic Research, Clinical & Experimental 2002;63(6):355‐365.
Malmstrom 1999 {published data only}
-
- Malmstrom K, Rodriguez‐Gomez G, Guerra J, Villaran C, Pineiro A, Lynn X, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Annals of Internal Medicine 1999;130(6):487‐95. - PubMed
Margolskee 1991 {published data only}
-
- Margolskee D, Bodman S, Dockhorn R, Irael E, Kemp J, Mansmann H. The therapeutic effects of MK‐571, a potent and selective leukotriene (LT) D4 receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1991;87(1 Part 2):309, Abs. 677.
Meltzer 2002 {published data only}
-
- Meltzer E O, Lockey R F, Friedman B F, Kalberg C, Goode‐Sellers S, Srebro S, Edwards L, Rickard K. Efficacy and safety of low‐dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clinic Proceedings 2002;77(5):437‐445. - PubMed
Micheletto 1997 {unpublished data only}
-
- Micheletto C, Turco P, Dal Negro R. Accolate 20 mg works as steroid sparing in moderate asthma. American Journal of Respiratory & Critical Care Medicine 1997; Vol. 155, issue 4 Part 2:A664.
Minkwitz 1998 {published data only}
-
- Minkwitz MC t al. Zafirlukast (Accolate) response in severe persistent ?. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. - PubMed
Miyamoto 1999 {published data only}
-
- Miyamoto T, Accolate™ clinical trial committee. Effects of zafirlukast on symptoms and pulmonary function of asthmatic patients with and without corticosteroids. European Respiratory Society 1999:P835.
Nathan 1998 {published data only}
-
- Nathan RA, Bernstein JA, Bielory L, Bonuccelli CM, Calhoun WJ, Galant SP, et al. Zafirlukast improves asthma symptoms and quality of life in patients with moderate reversible airflow obstruction. Journal of Allergy & Clinical Immunology 1998;102:935‐42. - PubMed
Nathan 2001 {published data only}
-
- Nathan RA, Bleecker ER, Kalberg C. A comparison of short‐term treatment with inhaled fluticasone propionate and zafirlukast for patients with persistent asthma. American Journal of Medicine 2001;111(3):195‐202. - PubMed
Nelson 2001 {published data only}
-
- Nelson H S, Nathan R A, Kalberg C, Yancey S W, Rickard K, A. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medgenmed Computer File: Medscape General Medicine 2001;3(4):3. - PubMed
Nishizawa 2002 {published data only}
-
- Nishizawa Y, Greicy‐Goto H, Tanigaki Y, Fushiki S. Sparing effect of Saibokuto inhalation on inhaled beclomethasone dipropionate to halved of reduction of inhaled beclomethasone dipropionate‐dose: Well‐controlled comparative study of Saiboku‐to‐inhalation and sodium cromoglycate‐inhalation. Japanese. Oto‐Rhino‐Laryngology Tokyo 2002;45(SUPPL. 1):8‐15.
Noonan 1998 {published data only}
-
- Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, et al. Montelukast, a potent leukotriene recpetor antagonist, causes dose‐related improvements in chronic asthma. European Respiratory Journal 1998;11:1232‐9. - PubMed
Nsouli 2001 {published data only}
-
- Nsouli SM, McNutt WJ. The additive effects of montelukast and salmeterol in moderate asthmatics who are uncontrolled on a low dose on inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001; 86:81.;86:81.
O'Shaughnessy 1996 {published data only}
Obase 2001 {published data only}
-
- Obase Y, Shimoda T, Tomari S, Mitsuta K, Fukushima C, et al. Efficacy and safety of long‐term treatment of athma in patients with pranlukast, a cysteinyl‐leukotriene‐receptor antagonist: a four‐year follow‐up study. Annals of Allergy, Asthma, & Immunology 2001;87(1):43‐7. - PubMed
Overbeek 2002 {published data only}
-
- Overbeek SE, O'Sullivan S, Leman K, Mulder PGM, Hoogsteden, Prins JB. Treatment with montelukast is less effective in reducing eosinophilic airway inflammation than fluticasone propionate in atopic asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215.
Paterson 1999 {published data only}
-
- Paterson MC, Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. The effect of combination therapy with salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society 1999:P3490.
Pearlman 1999 {published data only}
-
- Pearlman DS, Ostrom NK, Bronsky EA, Bonuccelli CM, Hanby LA. The leukotriene D4‐receptor antagonist zafirlukast attenuates exercise‐induced bronchoconstriction in children. Journal of Pediatrics 1999;134(3):273‐9. - PubMed
Pearlman 2002 {published data only}
-
- Pearlman D S, White M V, Lieberman A K, Pepsin P J, Kalberg C, Emmett A, Bowers B, Rickard K A, Dorinsky P. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Ann Allergy Asthma Immunol 2002;88(2):227‐35. - PubMed
Pizzichini 1999 {published data only}
-
- Pizzichini E, Leff JA, Reiss TF, Hendeles L, Boulet L‐P, Wei LX, Efthimiadis A, et al. Montelukast reduced airway eosinophilic inflammation in asthma: a randomized, controlled trial. European Respiratory Journal 1999;14(1):12‐28. - PubMed
Pullerits 1999 {published data only}
-
- Pullerits T, Praks L, Skoogh BE, Ani R, Lotvall J. Randomized placebo‐controlled study comparing a leukotriene receptor antagonist and a nasal glucocorticoid in seasonal allergic rhinitis. American Journal of Respiratory & Critical Care Medicine 1999;159(6):1814‐8. - PubMed
Pullerits 2001 {published data only}
-
- Pullerits T, Praks L, Baker R, Ani R, Lotvall J. Comparison of nasal fluticasone propionate, montelukast, and combined montelukast+loratadine in allergic rhinitis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A193.
Pullerits 2002 {published data only}
-
- Pullerits T, Praks L, Ristioja V, Lotvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. Journal of Allergy & Clinical Immunology 2002;109(6):949‐955. - PubMed
Ramsay 1997 {published data only}
-
- Ramsay CF, Kan CI, Nieman RB, Wang J, Krieken JHJM, Willems LNA, et al. The effects of oral pranlukast on airway immunopathology and clinical parameters in patients with asthma. American Thoracic Society. 1997:Abs C21.
Ramsay 1998 {published data only}
-
- Ramsay CF, Kan CI, Sterk PJ, Barnes NC. Pranlukast improves spirometry and bronchial hyperresponsiveness (BHR) in patients with mild asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Reiss 1996 {published data only}
-
- Reiss TF, Altman LC, Chervinsky P, Bewtra A, Stricker WE, Noonan GP, et al. Effects of montelukast (MK‐0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1996;98:528‐34. - PubMed
Reiss 1997a {published data only}
-
- Reiss TF, et al. Montelukast improves asthma outcomes over a 3‐month treatment period. American Thoracic Society. 1997.
Reiss 1997b {published data only}
Reiss 1998a {published data only}
-
- Reiss TF, White R, Noonan G, Korenblat P, Hess J, Shingo S. Montelukast (MK‐0476) a cys LT1 receptor antagonist improves the signs and symptoms of asthma over one year of treatment. Allergy & Asthma Proceedings 1998;19(4):205‐6.
Reiss 1998b {published data only}
-
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once‐daily leukotriene receptor antagonist, in the treatment of chronic asthma. Archives of Internal Medicine 1998;158:1213‐20. - PubMed
Reiss 1998c {published data only}
-
- Reiss TF, White R, Noonan G, Korenblat P, Hess J, Shingo S. Montelukast (MK‐0476) a cys LT1 receptor antagonist improves the signs and symptoms of asthma over one year of treatment. European Respiratory Journal 1997;10 Suppl 25:437s.
Riccioni 2002 (B) {published data only}
-
- Riccioni G, D'Orazio N, Ilio C, Della Vecchia R, Lorenzo A. Effectiveness and safety of montelukast versus budesonide at different doses on bronchial reactivity in subjects with mild‐persistent asthma. Clinica Terapeutica 2002;153(5):317‐321. - PubMed
Riccioni 2002 (c) {published data only}
-
- Riccioni G, Ballone E, D'Orazio N, Sensi S, Nicola M, Mascio R, Santilli F, Guagnano M T, Della Vecchia R. Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology & Pharmacology 2002;15(2):149‐155. - PubMed
Ringdal 1997 {published data only}
-
- Ringdal N, Whitney JG, Summerton L. Problems with inhaler technique and patient preference for oral therapy tablet zafirlukast versus inhaled beclomethasone. European Respiratory Journal 1997;10(Suppl 25):4372, abs P2806.
Robinson 2001 {published data only}
-
- Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double‐blind placebo‐controlled trial. Lancet 2001;357(9273):2007‐11. - PubMed
Rosenhall 2003 {published data only}
-
- Rosenhall L, Elvstrand A, Tilling B, Vinge I, Jemsby P, Stahl E, Jerre F, Bergqvist P B F. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler ) for the treatment of asthma. Respiratory Medicine 2003;97(6):702‐708. - PubMed
Sahn 1997 {published data only}
-
- Sahn SA, Galant S, Murray J, Bronsky E, Spector S, Faiferman I, et al. Pranlukast (Ultair) improves FEV in patients with asthma: results of a 12‐week multicenter study versus nedocromil. American Thoracic Society. 1997:Abs C49.
Schwartz 1998 {published data only}
-
- Schwartz HJ, Petty T, Dube LM, Swanson LJ, Lancaster JF. A randomized controlled trial comparing zileuton with theophylline in moderate asthma. Archives of Internal Medicine 1998;158(2):141‐8. - PubMed
Sheth 2002 {published data only}
-
- Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost‐Effectiveness Comparison of Salmeterol/Fluticasone Propionate versus Montelukast in the Treatment of Adults with Persistent Asthma. Pharmacoeconomics 2002;20(13):909‐918. - PubMed
Skalky 1999 {published data only}
-
- Skalky CS, Edelman JM, Polis A, Bird S, Gormley GJ, Israel E. Montelukast sodium (MK) compared to inhaled beclomethasone dipropionate (BD) in adult asthmatics: a randomized, clinical trial. Journal of Allergy & Clinical Immunology 1999;103(1 Part 2):Abs. 880, protocol # 070.
Smith 1993 {published data only}
-
- Smith LJ, Glass M, Minkwitz MC. Inhibition of leukotriene D4‐induced bronchoconstriction in subjects with asthma: a concentration‐effect study of ICI 204,219. Clinical Pharmacology & Therapeutics 1993;54(4):430‐6. - PubMed
Smith 1998 {published data only}
-
- Smith LJ, Hanby LA, Lavins BJ, Simonson SG. A single dose of zafirlukast reduces LTD4‐induced bronchoconstriction in patients on maintenance inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 1998;81:43‐9. - PubMed
Spector 1992 {published data only}
-
- Spector SL, Glass M, Minkwitz MC, ICI Asthma Trial Group. The effect of six weeks of therapy with oral doses of ICI 204,219 in asthmatics. American Review of Respiratory Disease 1992;145(4):A16.
Spector 1994 {published data only}
-
- Spector SL, Smith LJ, Glass M. Effects of 6 weeks of therapy with oral doses of ICI 204,219, a leukotriene D4 antagonist, in subjects with bronchial asthma. American Journal of Respiratory & Critical Care Medicine 1994;150:618‐23. - PubMed
Spector 1995 {published data only}
-
- Spector S, Miller CJ, Glass M. 13‐week dose‐response study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379.
Stanford 2002 {published data only}
-
- Stanford R H, Borker R, Dorinsky P, Pepsin P, Kalberg C, Emmett A, Rickard K. The costs and efficacy of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with persistent asthma. Chest 2002:P422.
Stelmach 2002 {published data only}
-
- Stelmach I, Grzelewski T, Stelmach W, Majak P, Jerzynska J, Gorski P, Kuna P. Effect of triamcinolone acetonide, montelukast, nedocromil sodium and formoterol on eosinophil blood counts, ECP serum levels and clinical progression of asthma in children. Polski Merkuriusz Lekarski 2002;12(69):208‐213. - PubMed
Stelmach 2002 (b) {published data only}
-
- Stelmach I, Jerzynska J, Kuna P. A randomized, double‐blind trial of the effect of glucocorticoid, antileukotriene and [beta]‐agonist treatment on IL‐10 serum levels in children with asthma. Clinical & Experimental Allergy 2002;32(2):264‐269. - PubMed
Storms 2001 {published data only}
-
- Storms W, Michele T M, Knorr B, Noonan G, Shapiro G, Zhang J, Shingo S, Reiss T F. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clinical & Experimental Allergy 2001;31(1):77‐87. - PubMed
Suissa 1997 {published data only}
-
- Suissa S, Dennis R, Ernst P, Sheehy O, Wood‐Dauphinee S. Effectiveness of the leukotriene receptor antagonist zafirlukast for mild‐to‐moderate asthma: a randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1997;126(3):177‐83. - PubMed
Svensson 1994 {published data only}
-
- Svensson C, Greiff L, Andersson M, Alkner U, Persson CGA. Bradykinin‐, leukotriene D4‐, and histamine‐induced mucosal exudation of plasma in human airways in vivo. Abbott Laboratories 1994:303.
Tashkin 1998 {published data only}
-
- Tashkin DP, Minkwitz MC, Bonuccelli CM. Zafirlukast (Accolate) treatment results in better asthma control in patients with more moderate disease. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411.
Terzano 2001 {published data only}
-
- Terzano C, Allegra L, Barkai L, Cremonesi G. Beclomethasone dipropionate versus budesonide inhalation suspension in children with mild to moderate persistent asthma. European Review for Medical & Pharmacological Sciences 2001. - PubMed
Townley 1995 {published data only}
-
- Townley R, Glass M, Minkwitz MC. 6‐week, dose‐escalation study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379. - PubMed
Tukiainen 2002 {published data only}
-
- Tukiainen H, Rytila P, Hamalainen K M, Silvasti M S L, Keski‐Karhu J. Safety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid‐treated asthmatic patients. Respiratory Medicine 2002;96(4):221‐229. - PubMed
Verhoeven 2001 {published data only}
Vethanayagam 2002 {published data only}
-
- Vethanayagam D, Leigh R, Yoshida M, Watson RM, Rerecich T, Killian K, O'Byrne PM. Effects of montelukast and budesonide alone and together on allergen‐induced airway inflammation in asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216. - PubMed
Vidal 2001 {published data only}
-
- Vidal C, Fernandez‐Ovide E, Pineiro J, et al. Comparison of montelukast versus budesonide in the treatment of exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 2001;86(6):655‐8. - PubMed
Volovitz 1999 {published data only}
-
- Volovitz B, Tabachnik E, Nussinovitch M, Shtaif B, Blau H, Gii‐Ad I, et al. Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma. Journal of Allergy & Clinical Immunology 1999;104(6):1162‐7. - PubMed
Von Berg 2002 {published data only}
-
- Berg A, Albrecht B, Darlath W, Vo H W, Berdel D. Intraindividual, randomised, double‐blind comparison between sodium cromoglycate and reproterol to assess the protective effect of the single drugs and their combination in children with exercise‐induced asthma. Allergologie 2002;25(11):557‐564.
Wahedna 1991 {published data only}
Weinberg 1998 {published data only}
-
- Weinberg EG, Summerton L, Harris A. Assessment of preference for oral zafirlukast vs inhaled beclomethasone in adolescent asthmatics. ERS Annual Congress. 1998; Vol. 12, issue 28:abs P0333.
Welch 1994 {published data only}
-
- Welch MJ, Nelson HS, Paull BR, Smith JA, Feiss G, Tobey RE. Effect of RG 12525, a new leukotriene antagonist, on pulmonary function of asthmatic adults. Annals of Allergy 1994;72:348‐52. - PubMed
Wenzel 1994 {published data only}
-
- Wenzel S, Cohn J, Trudeau J, Wilson W, Martin R, Westcott J. Zileuton (Leutrol) decreases urine LTE4, BALF LTB4 and improves lung function in nocturnal asthmatics. Abbott Laboratories 1994:302.
Wenzel 1995 {published data only}
-
- Wenzel SE, Trudeau JB, Kaminsky DA, Cohn J, Martin RJ, Westcott JY. Effect of 5‐lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. American Journal of Respiratory & Critical Care Medicine 1995;152(3):897‐905. - PubMed
Wenzel 1997 {published data only}
-
- Wenzel S, Chervinsky P, Kerwin E, Silvers W, Faiferman I, Dubb J. Oral pranlukast (Ultair) vs. inhaled beclomethasone: results of a 12‐week trial in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1997;155:A203.
Westbroek 1998 {published data only}
-
- Westbroek J, Pasma HR, James MH, Thwaites RMA. Inhaled fluticasone propionate and oral zafirlukast in moderate asthma: a clinical and cost comparison [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A416.
Westbroek 2000 {published data only}
-
- Westbroek J, Pasma HR. Effects of 2 weeks of treatment with fluticasone propionate 100 mcg b.d. by comparison with zafirlukast 20 mg b.d. on bronchial hyper‐responsiveness in patients with mild to moderate asthma. Respiratory Medicine 2000;94(2):112‐8. - PubMed
Williams 2000 {published data only}
-
- Williams B, Noonan G, Reiss TF, Knorr B, et al. Long‐term asthma control with oral montelukast and inhaled beclomethasone. Clinical & Experimental Allergy 2001;31:1‐10. - PubMed
Wilson 1999 {unpublished data only}
-
- Wilson AM. Comparative effects of oral montelukast and cetirizine versus intranasal mometasone furoate therapy in patients with seasonal allergic rhinitis. Ongoing trial 1999.
Wilson 1999 (b) {published data only}
-
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of salmeterol and montelukast as second‐line therapy in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society 1999:P3486.
Wilson 2001 {published data only}
-
- Wilson AM, Sims EJ, Orr LC, Coutie WJ, White PS, Gardiner Q, Lipworth BJ. Effects of topical corticosteroid and combined mediator blockade on domiciliary and laboratory measurements of nasal function in seasonal allergic rhinitis. Annals of Allergy, Asthma, & Immunology 2001;87(4):344‐349. - PubMed
Wilson 2001 (c) {published data only}
-
- Wilson A M, Orr L C, Sims E J, Lipworth B J. Effects of monotherapy with intra‐nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clinical & Experimental Allergy 2001;31(1):61‐68. - PubMed
Wilson 2001a {published data only}
-
- Wilson Am, Orr LC, Sims EJ, Dempsey OJ. Antiasthmatic effects of mediator block versus topical corticosteroids in allergic rhinitis and asthma. American Journal of Respiratory & Critical Care Medicine 2001;162 (4 part 1):1297‐1301. - PubMed
Wilson 2001b {published data only}
-
- Wilson Am, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second‐line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119(4):1021‐6. - PubMed
Xiang 2001 {published data only}
-
- Xiang X D, Zhou R, Chen Y. Influence of zafirlukast on pulmonary functions and quality of life in patients with asthma. Bulletin of Hunan Medical University 2001;26(3):251‐253. - PubMed
Yamamoto 1994 {published data only}
-
- Yamamoto H, Nagata M, Kuramitsu K, Tabe K, Kiuchi H, Sakamoto Y, et al. Inhibition of analgesic‐induced asthma by leukotriene receptor antagonist ONO‐1078. American Journal of Respiratory & Critical Care Medicine 1994;150:254‐7. - PubMed
Yamauchi 2001 {published data only}
-
- Yamauchi K, Tanifuji Y, Pan LH, et al. Effects of pranlukast, a leukotriene receptor antagonist, on airway inflammation in mild asthmatics. Journal of Asthma 2001;38(1):51‐7. - PubMed
Yoo 2001 {published data only}
-
- Yoo SH, Park SH, Song JS, et al. Clinical effects of pranlukast, an oral leukotriene receptor antagonist, in mild‐to‐moderate asthma: a 4‐week randomized multicentre controlled trial. Respirology 2001;6(1):15‐21. - PubMed
Yoshida 2000 {published data only}
-
- Yoshida S, Sakamoto H, Ishizaki Y, Onuma K, Shoji T, Nakagawa H, Hasegawa H, Nakabayashi M, Amayasu H. Efficacy of leukotriene receptor antagonist in bronchial hyperresponsiveness and hypersensitivity to analgesic in aspirin‐intolerant asthma. Clinical & Experimental Allergy 2000;30(1):64‐70. - PubMed
Yoshida 2002 {published data only}
-
- Yoshida M, Vethanayagam D, Leigh R, Matsumoto K, Watson RM, Reerich T, Killian KJ, O'Bryne PM. A comparison montelukast and budesonide effects on interleukin‐10 profiles in peripheral blood lymphocytes. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217.
Zhang 1999 {unpublished data only}
-
- Zhang J, Chang Y, Reiss TF. Predicting future response using patient baseline variables and early responses to montelukast, a potent cysLT1 antagonist. Merck Research Laboratories 1999.
Zorc 2003 {published data only}
-
- Zorc J J, Scarfone R J, Li Y, Hong T, Harmelin M, Grunstein L, Andre J B. Scheduled follow‐up after a pediatric emergency department visit for asthma: A randomized trial. Pediatrics 2003;111(3):495‐502. - PubMed
References to studies awaiting assessment
Bilancia 2000 {published data only}
-
- Bilancia R, Margiotta D Balacco D. Low doses of budesonide (B) plus montelukast (M) versus high doses of budesonide in asthmatic patients [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A197.
Demuro‐Mercon 2001 {published data only}
-
- Demuro‐Mercon C, Turpin J, Santanello N, Firriolo K, Edelman J. Motelukast improves asthma quality of life when added to Fluticasone. Journal of Allergy and Clinical Immunology 2001;107(2):S248.
Li 2001 {published data only}
-
- Li J, Zhang DH, Yang JY. Evaluation of the therapeutic effects of zafirlukast in asthma patients during reduction of high‐dose inhaled corticosteroid. Journal of Clinical Lung Section 2001;6(4):9‐11.
References to ongoing studies
Barnes NC {unpublished data only}
-
- Barnes NC. A randomised multi‐centre, double‐blind study to evaluate the effect of adding montelukast sodium to inhaled budesonide compared to doubling the dose of inhaled budesonide in adult patients with asthma. Ongoing trial 2000.
Additional references
Anonymous 2001
-
- Anonymous. IND Safety Reports. Code of Federal Regulations‐ U.S.Government Printing Office via GPO Access 2001; Vol. Title 21,Volume 5; section 312.32:69‐71. Available from :http://www.fda.gov/cder/regulatory.
Australia 2002
-
- Asthma Management Handbook 2002. Vol. 5th edition, Melbourne: National Asthma Campaign, 2002.
Beveridge 1996
Bootsma 1997
-
- Bootsma GP, Dekhuijzen PR, Festen J, Vanherwaarden CA. Effects of inhaled corticosteroids on bone [Review]. Netherlands Journal of Medicine 1997;50(6):254‐60. - PubMed
Boulet 2001
-
- Boulet LP, Bai TR, Becker A, Berube D, Beveridge R, Bowie DM, et al. What is new since the last (1999) Canadian Asthma Consensus Guidelines?. Can Respir J 2001;8(Suppl A):5a‐27a. - PubMed
BTS 2003
Cates 2002
Cook 1993
-
- Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta‐analyses? Current convictions and controversies. The Journal of the American Medical Association 1993;269:2749‐53. - PubMed
CTS 1999
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Ducharme 2001
-
- Ducharme FM, Hicks G. Anti‐leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma (Cochrane Review). The Cochrane Library 2001, Issue 2. - PubMed
Efthimiou 1998
-
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth [Review]. The European Respiratory Journal 1998;11(5):1167‐77. - PubMed
Egger 1997
GINA 2002
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. NIH Publication 02‐3659. Bethesda, MD: National Heart, Lung and Blood Institute, 2002.
Greenland 1985
-
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. - PubMed
Jadad 1995
-
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: Is blinding necessary?. Controlled Clinical Trials 1995;134(17):1‐12. - PubMed
Kamada 1995
-
- Kamada AK, Szefler SJ. Glucocorticoids and growth in asthmatic children [Review]. Pediatric Allergy & Immunology 1995;6(3):145‐54. - PubMed
Khan 1996
-
- Khan KS, Daya S, Jahad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. - PubMed
Mitchell 1992
-
- Mitchell EA. Consensus on acute asthma management in children. Ad Hoc Paediatric Group. The New Zealand Medical Journal 1992;105(941):353‐5. - PubMed
Murphy 1993
-
- Murphy S, Kelly HW. Asthma, inflammation, and airway hyperresponsiveness in children. [Review]. Current Opinion in Pediatrics 1993;5(3):255‐65. - PubMed
Piper 1989
-
- Piper PJ. Leukotrienes and the airways. European Journal of Anaesthesiology 1989;6:241‐55. - PubMed
Price 2000
-
- Price D. Tolerability of montelukast. Drugs 2000;59(suppl 1):35‐42. - PubMed
Rachelefsky 1993
-
- Rachelefsky GS, Warner JO. International consensus on the management of pediatric asthma: a summary statement. [Review]. Pediatric Pulmonology 1993;15(2):125‐7. - PubMed
Spahn 1996
-
- Spahn JD, Leung DYM. The role of glucocorticoids in the management of asthma. Allergy & Asthma Proceedings 1996;17(6):341‐50. - PubMed
Thornton 2000
-
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53:207‐16. - PubMed
USA 2002
-
- National Asthma Education and Prevention Program. NAEPP Expert Panel Report Guidelines for the Diagnosis and Management of Asthma. NIH Publication 02‐5075. National Heart, Lung and Blood Institute. Bethesda, MD: National Institute of Health, 2002:1‐111.
Wolthers 1996
-
- Wolthers OD. Long‐, intermediate‐ and short‐term studies in asthmatic children treated with inhaled glucocorticoids [Review]. European Respiratory Journal 1996;9(4):821‐7. - PubMed
Wolthers 1998
-
- Wolthers OD, Honour JW. Hypothalamic‐pituitary‐adrenal function in children with asthma and rhinitis treated with topical glucocorticoids [Review]. Clinical & Experimental Allergy 1998;28(5):545‐50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

