The stereospecificity and catalytic efficiency of the tryptophan synthase-catalysed exchange of the alpha-protons of amino acids
- PMID: 15107013
- PMCID: PMC1133895
- DOI: 10.1042/BJ20040388
The stereospecificity and catalytic efficiency of the tryptophan synthase-catalysed exchange of the alpha-protons of amino acids
Abstract
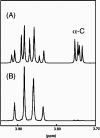
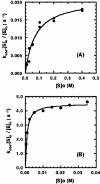
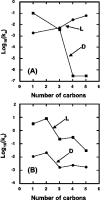
13C-NMR has been used to follow the tryptophan synthase (EC 4.2.1.20) catalysed hydrogen-deuterium exchange of the pro-2R and pro-2S protons of [2-13C]glycine at pH 7.8. 1H-NMR has also been used to follow the tryptophan-synthase-catalysed hydrogen-deuterium exchange of the alpha-protons of a range of L- and D-amino acids at pH 7.8. The pK(a) values of the alpha-protons of these amino acids have been estimated and we have determined whether or not their exchange rates can be predicted from their pK(a) values. With the exception of tryptophan and norleucine, the stereospecificities of the first-order alpha-proton exchange rates are independent of the size and electronegativity of the amino acid R-group. Similar results are obtained with the second-order alpha-proton exchange rates, except that both L-tryptophan and L-serine have much higher stereospecificities than all the other amino acids studied.
Figures

Similar articles
-
A comparative study of the kinetics and stereochemistry of the serine hydroxymethyltransferase- and tryptophan synthase-catalysed exchange of the pro-2R and pro-2S protons of glycine.Biochem J. 1991 Mar 15;274 ( Pt 3)(Pt 3):807-12. doi: 10.1042/bj2740807. Biochem J. 1991. PMID: 1849406 Free PMC article.
-
A substrate-induced change in the stereospecificity of the serine-hydroxymethyltransferase-catalysed exchange of the alpha-protons of amino acids--evidence for a second catalytic site.Eur J Biochem. 1998 Feb 15;252(1):113-7. doi: 10.1046/j.1432-1327.1998.2520113.x. Eur J Biochem. 1998. PMID: 9523719
-
Factors affecting the stereospecificity and catalytic efficiency of the tryptophan synthase-catalysed exchange of the pro-2R and pro-2S protons of glycine.Biochem J. 1995 Nov 1;311 ( Pt 3)(Pt 3):1015-9. doi: 10.1042/bj3111015. Biochem J. 1995. PMID: 7487918 Free PMC article.
-
Stereospecificity of alpha-proton exchange reactions catalysed by pyridoxal-5'-phosphate-dependent enzymes.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):138-42. doi: 10.1016/s1570-9639(03)00080-3. Biochim Biophys Acta. 2003. PMID: 12686123 Review.
-
Norvaline and norleucine may have been more abundant protein components during early stages of cell evolution.Orig Life Evol Biosph. 2013 Oct;43(4-5):363-75. doi: 10.1007/s11084-013-9344-3. Epub 2013 Sep 8. Orig Life Evol Biosph. 2013. PMID: 24013929 Review.
Cited by
-
An efficient protocol for the complete incorporation of methyl-protonated alanine in perdeuterated protein.J Biomol NMR. 2009 Feb;43(2):111-9. doi: 10.1007/s10858-008-9294-7. Epub 2008 Dec 30. J Biomol NMR. 2009. PMID: 19115043
References
-
- Malthouse J. P. G. Using NMR as a probe of protein structure and function. Biochem. Soc. Trans. 1999;27:701–713. - PubMed
-
- Malthouse J. P. G. Stereospecificity of α-proton exchange reactions catalysed by pyridoxal-5′-phosphate dependent enzymes. Biochim. Biophys. Acta. 2003;1647:138–142. - PubMed
-
- Fitzpatrick T. B., Malthouse J. P. G. Proof that serine hydroxymethyltransferase retains its specificity for the pro-2S proton of glycine in the absence of tetrahydrofolate. Biochem. Soc. Trans. 1996;24:132S. - PubMed
-
- Fitzpatrick T. B., Malthouse J. P. G. A substrate-induced change in the stereospecificity of the serine-hydroxymethyltransferase-catalysed exchange of the α-protons of amino acids – evidence for a second catalytic site. Eur. J. Biochem. 1998;252:113–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

