Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses
- PMID: 15107016
- PMCID: PMC1133779
- DOI: 10.1042/BJ20031506
Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses
Abstract
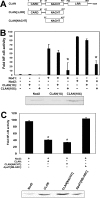
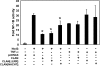
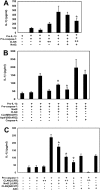
Proteins of the NACHT [NAIP (neuronal apoptosis inhibitory protein), CIITA (MHC class II transcription activator), HET-E (incompatibility locus protein from Podospora anserina) and TP1 (telomerase-associated protein)] family may serve as critical pathogen-sensing and signal-transducing molecules within the innate immune system. In the present paper, we show that CLAN [CARD (caspase-recruitment domain), LRR (leucine-rich repeat) and NACHT domain-containing protein], a NACHT-containing protein originally demonstrated to bind and activate pro-caspase 1, is also capable of influencing the functions of other members of the NACHT family. Through heterotypic NACHT-domain interactions, CLAN was found to associate with Nod1, Nod2 and NAC [nucleotide-binding domain and CARD-containing protein; NALP1 (NACHT, LRR and PYRIN protein 1)] when co-expressed in HEK-293T (human embryonic kidney) cells. NF-kappaB (nuclear factor kappaB) reporter assays demonstrated that co-expression of either full-length CLAN or the NACHT domain of CLAN significantly inhibited NF-kappaB activation induced by Nod1 or Nod2 overexpression. In addition, co-expression of CLAN or the NACHT domain of CLAN with Nod1 or Nod2 inhibited the ability of these proteins to generate active IL-1beta (interleukin 1beta) through their association with pro-caspase 1. The NACHT domain of CLAN was demonstrated by co-immunoprecipitation experiments to bind all NACHT domains that were tested, including the NACHT domains from CLAN itself, Nod1, Nod2, cryopyrin, NAC, PAN2 [PAAD [pyrin, AIM (absent-in-melanoma), ASC (apoptosis-associated speck-like protein containing a CARD) and death-domain-like]- and NACHT-containing protein] and NAIP (neuronal apoptosis inhibitory protein). Finally, monocyte-expressed CLAN was found to associate with Nod2 following exposure to bacterial peptidoglycan, implying a regulatory role for interaction of these NACHT proteins in the innate immune response. These studies suggest that by mediating hetero-oligomerization, NACHT domains provide a means by which various NACHT-containing proteins may interact, creating protein-interaction networks that potentially modulate immune responses to invading pathogens.
Figures





References
-
- Ogura Y., Bonen D. K., Inohara N., Nicolae D. L., Chen F. F., Ramos R., Britton H., Moran T., Karaliuskas R., Duerr R. H., et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature (London) 2001;411:603–606. - PubMed
-
- Miceli-Richard C., Lesage S., Rybojad M., Prieur A. M., Manouvrier-Hanu S., Hafner R., Chamaillard M., Zouali H., Thomas G., Hugot J. P. CARD15 mutations in Blau syndrome. Nat. Genet. 2001;29:19–20. - PubMed
-
- Li P., Allen H., Banerjee S., Franklin S., Herzog L., Johnston C., McDowell J., Paskind M., Rodman L., Salfeld J., et al. Mice deficient in IL-1β-converting enzyme are defective in production of mature IL-1β and resistant to endotoxic shock. Cell. 1995;80:401–411. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

