The Notch target genes Hey1 and Hey2 are required for embryonic vascular development
- PMID: 15107403
- PMCID: PMC395849
- DOI: 10.1101/gad.291004
The Notch target genes Hey1 and Hey2 are required for embryonic vascular development
Abstract
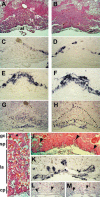
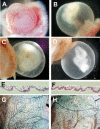
The Delta-Notch signaling pathway plays a central role in the development of most vertebrate organs. The Hey family of bHLH transcription factors are direct targets of Notch signaling. Loss of Hey2 in the mouse leads to cardiac defects with high postnatal lethality. We have now generated a mouse Hey1 knockout that has no apparent phenotypic defect. The combined loss of Hey1 and Hey2, however, results in embryonic death after embryonic day 9.5 (E9.5) with a global lack of vascular remodeling and massive hemorrhage. Initial vasculogenesis appears unaffected, but all subsequently developing major vessels in the embryo and yolk sac are either small or absent. Furthermore, the placental labyrinth completely lacks embryonic blood vessels. Similar vascular defects are observed in Jagged1 and Notch1 knockout mice. In the latter we found Hey1 and Hey2 expression in yolk sacs to be strongly reduced. Remaining large arteries in both Notch1 and Hey1/Hey2 knockout mice fail to express the arterial endothelial markers CD44, neuropilin1, and ephrin-B2. This indicates that Hey1/Hey2 are essential transducers of Notch signals in cardiovascular development that may mediate arterial cell fate decision.
Figures


References
-
- Batlle, E., Henderson, J.T., Beghtel, H., van den Born, M.M., Sancho, E., Huls, G., Meeldijk, J., Robertson, J., van de Wetering, M., Pawson, T., et al. 2002. Beta-catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263. - PubMed
-
- Carmeliet, P. 2003. Angiogenesis in health and disease. Nat. Med. 9: 653–660. - PubMed
-
- Donovan, J., Kordylewska, A., Jan, Y.N., and Utset, M.F. 2002. Tetralogy of fallot and other congenital heart defects in Hey2 mutant mice. Curr. Biol. 12: 1605–1610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
