Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells
- PMID: 15107499
- PMCID: PMC406435
- DOI: 10.1073/pnas.0307306101
Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells
Abstract
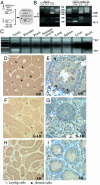
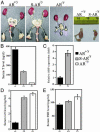
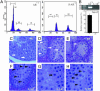
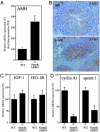
Androgens and the androgen receptor (AR) play important roles in male fertility, although the detailed mechanisms, particularly how androgen/AR influences spermatogenesis in particular cell types, remain unclear. Using a Cre-Lox conditional knockout strategy, we generated a tissue-specific knockout mouse with the AR gene deleted only in Sertoli cells (S-AR(-/y)). Phenotype analyses show the S-AR(-/y) mice were indistinguishable from WT AR mice (B6 AR(+/y)) with the exception of testes, which were significantly atrophied. S-AR(-/y) mice were infertile, with spermatogenic arrest predominately at the diplotene premeiotic stage and almost no sperm detected in the epididymides. S-AR(-/y) mice also have lower serum testosterone concentrations and higher serum leuteinizing hormone concentrations than B6 AR(+/y) mice. Further mechanistic studies demonstrated that S-AR(-/y) mice have defects in the expression of anti-Müllerian hormone, androgen-binding protein, cyclin A1, and sperm-1, which play important roles in the control of spermatogenesis and/or steroidogenesis. Together, our Sertoli cell-specific AR knockout mice provide in vivo evidence of the need for functional AR in Sertoli cells to maintain normal spermatogenesis and testosterone production, and ensure normal male fertility.
Figures

References
-
- Mackay, S. (2000) Int. Rev. Cytol. 200, 47-99. - PubMed
-
- Wolgemuth, D. J., Laurion, E. & Lele, K. M. (2002) Recent Prog. Horm. Res. 57, 75-101. - PubMed
-
- Jegou, B. (1993) Int. Rev. Cytol. 147, 25-96. - PubMed
-
- Roberts, K. P., Awoniyi, C. A., Santulli, R. & Zirkin, B. R. (1991) Endocrinology 129, 3417-3423. - PubMed
-
- Rivarola, M. A., Sanchez, P. & Saez, J. M. (1985) Endocrinology 117, 1796-1802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

