Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study
- PMID: 15108303
- PMCID: PMC6871891
- DOI: 10.1002/hbm.20025
Activation of cerebellar hemispheres in spatial memorization of saccadic eye movements: an fMRI study
Abstract
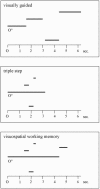
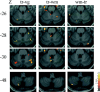
What mechanisms allow us to direct a precise saccade to a remembered target position in space? The cerebellum has been proposed to be involved not only in motor and oculomotor control, but also in perceptual and cognitive functions. We used functional MRI (Echoplanar imaging at 1.5 T) to investigate the role of the cerebellum in the control of externally triggered and internally generated saccadic eye movements of high and low memory impact, in six healthy volunteers. Memory-guided saccades to remembered locations of 3 targets (triple-step saccades) in contrast to either central fixation or to visually guided saccades activated the cerebellar hemispheres predominantly within lobuli VI-crus I. The same areas were activated when an analogous visuospatial working memory task was contrasted to the triple-step saccades. Visually guided saccades activated the posterior vermis and the triple-step saccades, contrasted to the working memory task, activated predominantly the posterior vermis and paravermal regions. Our data confirm the primary involvement of the posterior vermis for visually-triggered saccadic eye movements and present novel evidence for a role of the cerebellar hemispheres in the mnemonic and visuospatial control of memory-guided saccades.
Copyright 2004 Wiley-Liss, Inc.
Figures



Similar articles
-
The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements--an fMRI study.Prog Brain Res. 2005;148:151-64. doi: 10.1016/S0079-6123(04)48013-3. Prog Brain Res. 2005. PMID: 15661188 Review.
-
Positron emission tomography study of voluntary saccadic eye movements and spatial working memory.J Neurophysiol. 1996 Jan;75(1):454-68. doi: 10.1152/jn.1996.75.1.454. J Neurophysiol. 1996. PMID: 8822570
-
Cerebellar activation related to saccadic inaccuracies.Cerebellum. 2013 Apr;12(2):224-35. doi: 10.1007/s12311-012-0417-z. Cerebellum. 2013. PMID: 23055081
-
Differences in cortical activation during smooth pursuit and saccadic eye movements following cerebellar lesions.Exp Brain Res. 2007 Aug;181(2):237-47. doi: 10.1007/s00221-007-0922-3. Epub 2007 Mar 20. Exp Brain Res. 2007. PMID: 17372726
-
Cerebellar control of saccadic eye movements: its neural mechanisms and pathways.Jpn J Physiol. 1991;41(3):351-68. doi: 10.2170/jjphysiol.41.351. Jpn J Physiol. 1991. PMID: 1960885 Review.
Cited by
-
Distinct cerebellar regions for body motion discrimination.Soc Cogn Affect Neurosci. 2022 Feb 3;17(1):72-80. doi: 10.1093/scan/nsz088. Soc Cogn Affect Neurosci. 2022. PMID: 31820788 Free PMC article.
-
The cerebellar dysplasia of Chiari II malformation as revealed by eye movements.Can J Neurol Sci. 2009 Nov;36(6):713-24. doi: 10.1017/s0317167100008325. Can J Neurol Sci. 2009. PMID: 19960749 Free PMC article.
-
An fMRI study of optokinetic nystagmus and smooth-pursuit eye movements in humans.Exp Brain Res. 2005 Aug;165(2):203-16. doi: 10.1007/s00221-005-2289-7. Epub 2005 Apr 29. Exp Brain Res. 2005. PMID: 15864563
-
Early Cerebellar Network Shifting in Spinocerebellar Ataxia Type 6.Cereb Cortex. 2016 Jul;26(7):3205-18. doi: 10.1093/cercor/bhv154. Epub 2015 Jul 24. Cereb Cortex. 2016. PMID: 26209844 Free PMC article.
-
Visuomotor cerebellum in human and nonhuman primates.Cerebellum. 2012 Jun;11(2):392-410. doi: 10.1007/s12311-010-0204-7. Cerebellum. 2012. PMID: 20809106 Free PMC article. Review.
References
-
- Allen G, Buxton RB, Wong EC, Courchesne E (1997): Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943. - PubMed
-
- Aschoff JC, Cohen B (1971): Changes in saccadic eye movements produced by cerebellar cortical lesions. Exp Neurol 32: 123–133. - PubMed
-
- Brodal P, Bjaalie JG (1992): Organization of the pontine nuclei. Neurosci Res 13: 83–118. - PubMed
-
- Büttner U (1999): Eye movements deficits in cerebellar disease In: Becker U, Deubel H, Mergner T, editors. Current oculomotor research. New York: Plenum Press; p 383–389.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

