The use of "overall accuracy" to evaluate the validity of screening or diagnostic tests
- PMID: 15109345
- PMCID: PMC1492250
- DOI: 10.1111/j.1525-1497.2004.30091.x
The use of "overall accuracy" to evaluate the validity of screening or diagnostic tests
Abstract
Objective: Evaluations of screening or diagnostic tests sometimes incorporate measures of overall accuracy, diagnostic accuracy, or test efficiency. These terms refer to a single summary measurement calculated from 2 x 2 contingency tables that is the overall probability that a patient will be correctly classified by a screening or diagnostic test. We assessed the value of overall accuracy in studies of test validity, a topic that has not received adequate emphasis in the clinical literature.
Design: Guided by previous reports, we summarize the issues concerning the use of overall accuracy. To document its use in contemporary studies, a search was performed for test evaluation studies published in the clinical literature from 2000 to 2002 in which overall accuracy derived from a 2 x 2 contingency table was reported.
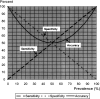
Measurements and main results: Overall accuracy is the weighted average of a test's sensitivity and specificity, where sensitivity is weighted by prevalence and specificity is weighted by the complement of prevalence. Overall accuracy becomes particularly problematic as a measure of validity as 1) the difference between sensitivity and specificity increases and/or 2) the prevalence deviates away from 50%. Both situations lead to an increasing deviation between overall accuracy and either sensitivity or specificity. A summary of results from published studies (N = 25) illustrated that the prevalence-dependent nature of overall accuracy has potentially negative consequences that can lead to a distorted impression of the validity of a screening or diagnostic test.
Conclusions: Despite the intuitive appeal of overall accuracy as a single measure of test validity, its dependence on prevalence renders it inferior to the careful and balanced consideration of sensitivity and specificity.
Figures


References
-
- Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113–34. - PubMed
-
- Begg CB. Biases in the assessment of diagnostic tests. Stat Med. 1987;6:411–23. - PubMed
-
- Metz CE. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–98. - PubMed
-
- Weiss N. 2nd ed. New York, NY: Oxford University Press; 1996. Clinical Epidemiology: The Study of the Outcome of Illness; pp. 20–1.
-
- Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002;359:881–4. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
