ACE2, a new regulator of the renin-angiotensin system
- PMID: 15109615
- PMCID: PMC7128798
- DOI: 10.1016/j.tem.2004.03.001
ACE2, a new regulator of the renin-angiotensin system
Abstract
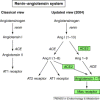
Angiotensin-converting enzyme (ACE) is a zinc metalloproteinase and a key regulator of the renin-angiotensin system (RAS). ACE2 is a newly described enzyme identified in rodents and humans with a more restricted distribution than ACE, and is found mainly in heart and kidney. ACE2 cleaves a single residue from angiotensin I (Ang I) to generate Ang 1-9, and degrades Ang II, the main effector of the RAS, to the vasodilator Ang 1-7. The importance of ACE2 in normal physiology and pathophysiological states is largely unknown. ACE2 might act in a counter-regulatory manner to ACE, modulating the balance between vasoconstrictors and vasodilators within the heart and kidney, and playing a significant role in regulating cardiovascular and renal function.
Figures
References
-
- Erdos E.G. Conversion of angiotensin I to angiotensin II. Am. J. Med. 1976;60:749–759. - PubMed
-
- Johnston C.I. Tissue angiotensin converting enzyme in cardiac and vascular hypertrophy, repair, and remodeling. Hypertension. 1994;23:258–268. - PubMed
-
- Dzau V.J. Tissue angiotensin and pathobiology of vascular disease – a unifying hypothesis. Hypertension. 2001;37:1047–1052. - PubMed
-
- Donoghue M. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. - PubMed
-
- Tipnis S.R. A human homolog of angiotensin-converting enzyme – cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 2000;275:33238–33243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

