Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope
- PMID: 15111310
- PMCID: PMC1615668
- DOI: 10.1016/S0002-9440(10)63722-4
Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope
Abstract
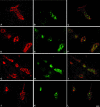
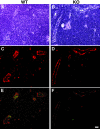
The interaction of L-selectin on lymphocytes with sulfated ligands on high endothelial venules (HEVs) of lymph nodes results in lymphocyte rolling and is essential for lymphocyte homing. The MECA-79 monoclonal antibody reports HEV-expressed ligands for L-selectin by recognizing a critical sulfation-dependent determinant on these ligands. HEC-GlcNAc6ST, a HEV-localized sulfotransferase, is essential for the elaboration of functional ligands within lymph nodes, as well as the generation of the MECA-79 epitope. Here, we use an antibody against murine HEC-GlcNAc6ST to study its expression in relationship to the MECA-79 epitope. In lymph nodes, the enzyme is expressed in the Golgi apparatus of high endothelial cells, in close correspondence with luminal staining by MECA-79. In lymph node HEVs of HEC-GlcNAc6ST-null mice, luminal staining by MECA-79 is almost abolished, whereas abluminal staining persists although reduced in intensity. HEV-like vessels in several examples of inflammation-associated lymphoid neogenesis, including nonobese diabetic mice, also exhibit concomitant expression of the sulfotransferase and luminal MECA-79 reactivity. The correlation extends to ectopic lymphoid aggregates within the pancreas of RIP-BLC mice, in which CXCL13 is expressed in islets. Analysis of the progeny of RIP-BLC by HEC-GlcNAc6ST-null mice establishes that the enzyme is responsible for the MECA-79 defined luminal ligands.
Figures

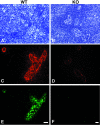
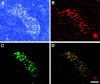
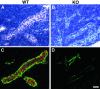
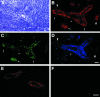
Similar articles
-
A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells.BMC Immunol. 2005 Mar 7;6:6. doi: 10.1186/1471-2172-6-6. BMC Immunol. 2005. PMID: 15752429 Free PMC article.
-
Lymphocyte-HEV interactions in lymph nodes of a sulfotransferase-deficient mouse.J Exp Med. 2003 Nov 3;198(9):1289-300. doi: 10.1084/jem.20030057. J Exp Med. 2003. PMID: 14597732 Free PMC article.
-
N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing.J Biol Chem. 2004 Aug 13;279(33):35001-8. doi: 10.1074/jbc.M404456200. Epub 2004 Jun 2. J Biol Chem. 2004. PMID: 15175329
-
Sulfated L-selectin ligands as a therapeutic target in chronic inflammation.Trends Immunol. 2006 Dec;27(12):559-65. doi: 10.1016/j.it.2006.10.007. Epub 2006 Oct 17. Trends Immunol. 2006. PMID: 17049924 Review.
-
Sulphated endothelial ligands for L-selectin in lymphocyte homing and inflammation.Biochem Soc Trans. 2003 Apr;31(2):313-7. doi: 10.1042/bst0310313. Biochem Soc Trans. 2003. PMID: 12653627 Review.
Cited by
-
High endothelial venules (HEVs) in immunity, inflammation and cancer.Angiogenesis. 2021 Nov;24(4):719-753. doi: 10.1007/s10456-021-09792-8. Epub 2021 May 6. Angiogenesis. 2021. PMID: 33956259 Free PMC article. Review.
-
A HEV-restricted sulfotransferase is expressed in rheumatoid arthritis synovium and is induced by lymphotoxin-alpha/beta and TNF-alpha in cultured endothelial cells.BMC Immunol. 2005 Mar 7;6:6. doi: 10.1186/1471-2172-6-6. BMC Immunol. 2005. PMID: 15752429 Free PMC article.
-
Transgenic LacZ under control of Hec-6st regulatory sequences recapitulates endogenous gene expression on high endothelial venules.Proc Natl Acad Sci U S A. 2007 Mar 13;104(11):4577-82. doi: 10.1073/pnas.0700334104. Epub 2007 Mar 5. Proc Natl Acad Sci U S A. 2007. PMID: 17360566 Free PMC article.
-
Therapeutic targeting of endothelial ligands for L-selectin (PNAd) in a sheep model of asthma.Am J Pathol. 2005 Mar;166(3):935-44. doi: 10.1016/S0002-9440(10)62313-9. Am J Pathol. 2005. PMID: 15743804 Free PMC article.
-
Genetic regulation of pristane-induced oil granuloma responses.Int J Exp Pathol. 2010 Oct;91(5):472-83. doi: 10.1111/j.1365-2613.2010.00732.x. Epub 2010 Aug 27. Int J Exp Pathol. 2010. PMID: 20804539 Free PMC article.
References
-
- Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. - PubMed
-
- Ruddle NH. Lymphoid neo-organogenesis: lymphotoxin’s role in inflammation and development. Immunol Res. 1999;19:119–125. - PubMed
-
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. - PubMed
-
- Rosen SD. Ligands for L-selectin: homing, inflammation and beyond. Ann Rev Immunol. 2004;22:129–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

