Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12)
- PMID: 15111311
- PMCID: PMC1615655
- DOI: 10.1016/s0002-9440(10)63723-6
Activation of the GLI oncogene through fusion with the beta-actin gene (ACTB) in a group of distinctive pericytic neoplasms: pericytoma with t(7;12)
Abstract
Activation of the GLI oncogene is an important step in the sonic hedgehog signaling pathway, and leads to, eg, tissue-specific cell proliferation during embryogenesis. GLI activity in adult tissues is restricted, but has been identified in various neoplasms, as a result of mutations in the PTCH (patched) or SMOH (smoothened) genes, encoding components of the sonic hedgehog pathway, or by amplification of GLI. Herein, we present a new mechanism of GLI activation through fusion with the beta-actin gene (ACTB) in five histologically distinctive soft tissue tumors showing a t(7;12)(p21-22;q13-15) and a pericytic phenotype. Each was composed of a perivascular proliferation of monomorphic short spindle cells that stained positively for smooth muscle actin and laminin and that showed pericytic features by electron microscopy. To date, with a median follow-up of 24 months, none has behaved in an aggressive manner. Molecular genetic analysis showed that the translocation in all cases resulted in a fusion transcript including the 5'-part of ACTB and the 3'-part of GLI. The DNA-binding zinc finger domains of GLI were retained in the fusion transcripts and it is likely that the replacement of the promoter region of GLI with that of the ubiquitously expressed ACTB gene leads to deregulation of GLI expression and its downstream target genes.
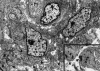
Figures





Similar articles
-
Molecular genetic characterization of the genomic ACTB-GLI fusion in pericytoma with t(7;12).Biochem Biophys Res Commun. 2004 Dec 24;325(4):1318-23. doi: 10.1016/j.bbrc.2004.10.172. Biochem Biophys Res Commun. 2004. PMID: 15555571
-
An Unbalanced Chromosome Translocation Between 7p22 and 12q13 Leads to ACTB-GLI1 Fusion in Pericytoma.Anticancer Res. 2020 Mar;40(3):1239-1245. doi: 10.21873/anticanres.14065. Anticancer Res. 2020. PMID: 32132020
-
A Distinct Malignant Epithelioid Neoplasm With GLI1 Gene Rearrangements, Frequent S100 Protein Expression, and Metastatic Potential: Expanding the Spectrum of Pathologic Entities With ACTB/MALAT1/PTCH1-GLI1 Fusions.Am J Surg Pathol. 2018 Apr;42(4):553-560. doi: 10.1097/PAS.0000000000001010. Am J Surg Pathol. 2018. PMID: 29309307 Free PMC article.
-
Gli-type zinc finger proteins as bipotential transducers of Hedgehog signaling.Differentiation. 2002 May;70(2-3):69-76. doi: 10.1046/j.1432-0436.2002.700201.x. Differentiation. 2002. PMID: 12076333 Review.
-
Therapeutic targeting of the Hedgehog-GLI pathway in prostate cancer.Cancer Res. 2005 Apr 15;65(8):2990-2. doi: 10.1158/0008-5472.CAN-05-0439. Cancer Res. 2005. PMID: 15833820 Review.
Cited by
-
[Abdominal soft tissue tumors].Pathologie (Heidelb). 2022 Aug;43(Suppl 1):42-49. doi: 10.1007/s00292-022-01128-7. Epub 2022 Oct 12. Pathologie (Heidelb). 2022. PMID: 36222918 Free PMC article. Review. German.
-
GLI1-amplifications expand the spectrum of soft tissue neoplasms defined by GLI1 gene fusions.Mod Pathol. 2019 Nov;32(11):1617-1626. doi: 10.1038/s41379-019-0293-x. Epub 2019 Jun 12. Mod Pathol. 2019. PMID: 31189998 Free PMC article.
-
Novel MIR143-NOTCH fusions in benign and malignant glomus tumors.Genes Chromosomes Cancer. 2013 Nov;52(11):1075-87. doi: 10.1002/gcc.22102. Epub 2013 Sep 2. Genes Chromosomes Cancer. 2013. PMID: 23999936 Free PMC article.
-
Molecularly defined sinonasal malignancies: an overview with focus on the current WHO classification and recently described provisional entities.Virchows Arch. 2024 Jun;484(6):885-900. doi: 10.1007/s00428-024-03775-y. Epub 2024 Mar 16. Virchows Arch. 2024. PMID: 38491228 Free PMC article. Review.
-
The value of GLI1 and p16 immunohistochemistry in the premolecular screening for GLI1-altered mesenchymal neoplasms.Virchows Arch. 2024 May;484(5):765-775. doi: 10.1007/s00428-023-03687-3. Epub 2023 Nov 8. Virchows Arch. 2024. PMID: 37940743 Free PMC article.
References
-
- Fletcher CDM, Rydholm A, Singer S, Sundaram M, Coindre JM. Soft tissue tumours: epidemiology, clinical features, histopathological typing and grading. Fletcher CDM, Unni KK, Mertens F, editors. Lyon: IARC Press; World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. 2002:pp 12–18.
-
- Åman P. Fusion genes in solid tumors. Semin Cancer Biol. 1999;9:303–318. - PubMed
-
- Ladanyi M, Bridge JA. Contribution of molecular genetic data to the classification of sarcomas. Hum Pathol. 2000;31:532–538. - PubMed
-
- Perez-Atayde AR, Kozakewich HWP, McGill T, Fletcher JA. Hemangiopericytoma of the tongue in a 12-year-old child: ultrastructural and cytogenetic observations. Hum Pathol. 1994;25:425–429. - PubMed
-
- Mandahl N. Methods in solid tumor cytogenetics. Rooney DE, editor. New York: Oxford University Press; Human CytogeneticsMalignancy and Acquired Abnormalities. (ed 3) 2001:pp 165–203.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

