Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells
- PMID: 15111328
- PMCID: PMC1615640
- DOI: 10.1016/S0002-9440(10)63740-6
Persistent proteinuria up-regulates angiotensin II type 2 receptor and induces apoptosis in proximal tubular cells
Abstract
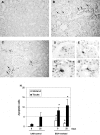
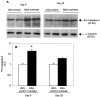
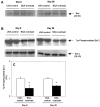
Apoptosis is implicated in the progressive cell loss and fibrosis both at glomerular and tubulointerstitial level. In this study, we examined the potential mechanisms by which persistent proteinuria (protein-overload model) could induce apoptosis. After uninephrectomy (UNX), Wistar rats received daily injections of 0.5 g of bovine serum albumin (BSA)/100 g body weight or saline. Both at day 8 and day 28, rats receiving BSA had proteinuria and renal lesions characterized by tubular atrophy and/or dilation and mononuclear cell infiltration. In relation to control-UNX rats, renal cortex of nephritic rats showed an increment in AT2 mRNA (reverse transcriptase-polymerase chain reaction) and protein (Western blot) expression. In both groups, AT2 receptor immunostaining was mainly localized in proximal tubular cells. Rats with persistent proteinuria showed a significantly increased number of terminal dUTP nick-end labeling positive apoptotic cells compared with UNX-controls, both in glomeruli and tubulointerstitium. Double staining for apoptosis and AT2 receptor showed that most terminal dUTP nick-end labeling positive cells were found in tubules expressing AT2 receptor. Using an antibody that recognizes the active form caspase-3, we observed an increment in caspase-3 activation in rats receiving BSA with respect to those receiving saline. Rats with persistent proteinuria showed a diminution in the phosphorylation of Bcl-2 with respect to UNX-controls both at day 8 and day 28. By contrast, no changes were observed either in the Bax or in the Bcl-2 protein levels. The administration of BSA to UNX rats induced a diminution in the phosphorylation of ERK with respect to UNX-control at all times studied. The changes observed in ERK activities took place without alterations of ERK1/2 protein levels. In summary, our data suggest that persistent proteinuria causes apoptosis in tubular cells through the activation of AT2 receptor, which can, in turn, inhibit MAP kinase (ERK1/2) activation and Bcl-2 phosphorylation.
Figures






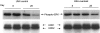
References
-
- Remuzzi G, Ruggenenti P, Benigni A. Understanding the nature of renal disease progression. Kidney Int. 1997;51:2–15. - PubMed
-
- Ortiz A, Lorz C, Catalán MP, Justo P, Egido J. Role and regulation of apoptotic cell death in the kidney. Y2K update. Front Biosci. 2000;5:D735–D749. - PubMed
-
- Thomas ME, Brunskill NJ, Harris KPG, Bailey E, Pringle JH, Furness PN, Walls J. Proteinuria induces tubular cell turnover: a potential mechanism for tubular atrophy. Kidney Int. 1999;55:890–898. - PubMed
-
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

