The facilitated probability of quantal secretion within an array of calcium channels of an active zone at the amphibian neuromuscular junction
- PMID: 15111388
- PMCID: PMC1304140
- DOI: 10.1016/S0006-3495(04)74323-4
The facilitated probability of quantal secretion within an array of calcium channels of an active zone at the amphibian neuromuscular junction
Abstract
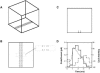
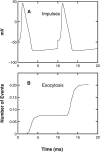
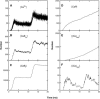
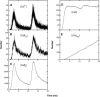
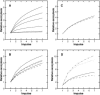
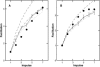
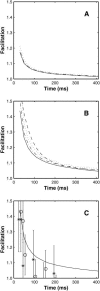
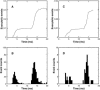
A Monte Carlo analysis has been made of the phenomenon of facilitation, whereby a conditioning impulse leaves nerve terminals in a state of heightened release of quanta by a subsequent test impulse, this state persisting for periods of hundreds of milliseconds. It is shown that a quantitative account of facilitation at the amphibian neuromuscular junction can be given if the exocytosis is triggered by the combined action of a low-affinity calcium-binding molecule at the site of exocytosis and a high-affinity calcium-binding molecule some distance away. The kinetic properties and spatial distribution of these molecules at the amphibian neuromuscular junction are arrived at by considering the appropriate values that the relevant parameters must take to successfully account for the experimentally observed amplitude and time course of decline of F1 and F2 facilitation after a conditioning impulse, as well as the growth of facilitation during short trains of impulses. This model of facilitation correctly predicts the effects on facilitation of exogenous buffers such as BAPTA during short trains of impulses. In addition, it accounts for the relative invariance of the kinetics of quantal release due to test-conditioning sequences of impulses as well as due to change in the extent of calcium influx during an impulse.
Figures




Similar articles
-
The probability of quantal secretion within an array of calcium channels of an active zone.Biophys J. 2000 May;78(5):2222-40. doi: 10.1016/S0006-3495(00)76770-1. Biophys J. 2000. PMID: 10777722 Free PMC article.
-
Mechanisms of calcium sequestration during facilitation at active zones of an amphibian neuromuscular junction.J Theor Biol. 2007 Jul 21;247(2):230-41. doi: 10.1016/j.jtbi.2007.03.022. Epub 2007 Mar 24. J Theor Biol. 2007. PMID: 17462674
-
The effects of calcium ions on the binomial parameters that control acetylcholine release during trains of nerve impulses at amphibian neuromuscular synapses.J Physiol. 1977 Oct;271(3):673-98. doi: 10.1113/jphysiol.1977.sp012020. J Physiol. 1977. PMID: 411920 Free PMC article.
-
Statistics of transmitter release at nerve terminals.Prog Neurobiol. 2000 Apr;60(6):545-606. doi: 10.1016/s0301-0082(99)00040-4. Prog Neurobiol. 2000. PMID: 10739089 Review.
-
The synaptic bouton acts like a salt shaker.Cell Biochem Biophys. 2004;41(2):259-64. doi: 10.1385/cbb:41:2:259. Cell Biochem Biophys. 2004. PMID: 15475612 Review.
Cited by
-
The number of components of enhancement contributing to short-term synaptic plasticity at the neuromuscular synapse during patterned nerve Stimulation progressively decreases as basal release probability is increased from low to normal levels by changing extracellular Ca2+.J Neurosci. 2011 May 11;31(19):7060-72. doi: 10.1523/JNEUROSCI.0392-11.2011. J Neurosci. 2011. PMID: 21562268 Free PMC article.
-
Transmitter release site organization can predict synaptic function at the neuromuscular junction.J Neurophysiol. 2018 Apr 1;119(4):1340-1355. doi: 10.1152/jn.00168.2017. Epub 2017 Dec 27. J Neurophysiol. 2018. PMID: 29357458 Free PMC article.
-
New insights into short-term synaptic facilitation at the frog neuromuscular junction.J Neurophysiol. 2015 Jan 1;113(1):71-87. doi: 10.1152/jn.00198.2014. Epub 2014 Sep 10. J Neurophysiol. 2015. PMID: 25210157 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

