Water dynamics and dewetting transitions in the small mechanosensitive channel MscS
- PMID: 15111405
- PMCID: PMC1304157
- DOI: 10.1016/S0006-3495(04)74340-4
Water dynamics and dewetting transitions in the small mechanosensitive channel MscS
Abstract
The dynamics of confined water in capillaries and nanotubes suggests that gating of ion channels may involve not only changes of the pore geometry, but also transitions between water-filled and empty states in certain locations. The recently solved heptameric structure of the small mechanosensitive channel of Escherichia coli, MscS, has revealed a relatively wide (7-15 A) yet highly hydrophobic transmembrane pore. Continuum estimations based on the properties of pore surface suggest low conductance and a thermodynamic possibility of dewetting. To test the predictions we performed molecular dynamics simulations of MscS filled with flexible TIP3P water. Irrespective to the initial conditions, several independent 6-ns simulations converged to the same stable state with the pore water-filled in the wider part, but predominantly empty in the narrow hydrophobic part, displaying intermittent vapor-liquid transitions. The polar gain-of-function substitution L109S in the constriction resulted in a stable hydration of the entire pore. Steered passages of Cl(-) ions through the narrow part of the pore consistently produced partial ion dehydration and required a force of 200-400 pN to overcome an estimated barrier of 10-20 kcal/mole, implying negligibly low conductance. We conclude that the crystal structure of MscS does not represent an open state. We infer that MscS gate, which is similar to that of the nicotinic ACh receptor, involves a vapor-lock mechanism where limited changes of geometry or surface polarity can locally switch the regime between water-filled (conducting) and empty (nonconducting) states.
Figures



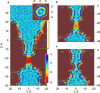
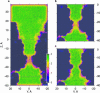
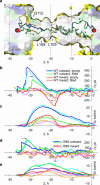

References
-
- Allen, R., S. Melchionna, and J. P. Hansen. 2002. Intermittent permeation of cylindrical nanopores by water. Phys. Rev. Lett. 89:175502. - PubMed
-
- Bass, R. B., P. Strop, M. Barclay, and D. C. Rees. 2002. Crystal structure of Escherichia coli MscS, a voltage-modulated and mechanosensitive channel. Science. 298:1582–1587. - PubMed
-
- Beckstein, O., P. C. Biggin, and M. S. P. Sansom. 2001. A hydrophobic gating mechanism for nanopores. J. Phys. Chem. B. 105:12902–12905.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

