Liquid domains in vesicles investigated by NMR and fluorescence microscopy
- PMID: 15111407
- PMCID: PMC1304159
- DOI: 10.1016/S0006-3495(04)74342-8
Liquid domains in vesicles investigated by NMR and fluorescence microscopy
Abstract
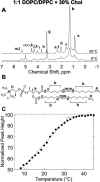
We use (2)H-NMR, (1)H-MAS NMR, and fluorescence microscopy to detect immiscibility in three particular phospholipid ratios mixed with 30% cholesterol: 2:1 DOPC/DPPC, 1:1 DOPC/DPPC, and 1:2 DOPC/DPPC. Large-scale (>>160 nm) phase separation into liquid-ordered (L(o)) and liquid-crystalline (L(alpha)) phases is observed by both NMR and fluorescence microscopy. By fitting superimposed (2)H-NMR spectra, we quantitatively determine that the L(o) phase is strongly enriched in DPPC and moderately enriched in cholesterol. Tie-lines estimated at different temperatures and membrane compositions are based on both (2)H-NMR observations and a previously published ternary phase diagram. (2)H- and (1)H-MAS NMR techniques probe significantly smaller length scales than microscopy experiments (submicron versus micron-scalp), and complex behavior is observed near the miscibility transition. Fluorescence microscopy of giant unilamellar vesicles shows micrometer-scale domains below the miscibility transition. In contrast, NMR of multilamellar vesicles gives evidence for smaller ( approximately 80 nm) domains just below the miscibility transition, whereas large-scale demixing occurs at a lower temperature, T(low). A transition at T(low) is also evident in fluorescence microscopy measurements of the surface area fraction of ordered phase in giant unilamellar vesicles. Our results reemphasize the complex phase behavior of cholesterol-containing membranes and provide a framework for interpreting (2)H-NMR experiments in similar membranes.
Figures








References
-
- Anderson, R. G., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. - PubMed
-
- Angelova, M. I., S. Soleau, P. Meleard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. Progr. Colloid Polym. Sci. 89:127–131.
-
- Aussenac, F., M. Tavares, and E. J. Dufourc. 2003. Cholesterol dynamics in membranes of raft composition: a molecular point of view from 2H and 31P solid-state NMR. Biochemistry. 42:1383–1390. - PubMed
-
- Baumgart, T., S. T. Hess, and W. W. Webb. 2003. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 425:821–824. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

