Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes
- PMID: 15111412
- PMCID: PMC1304164
- DOI: 10.1016/S0006-3495(04)74347-7
Role of cholesterol in the formation and nature of lipid rafts in planar and spherical model membranes
Abstract
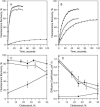
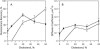
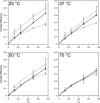
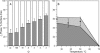
Sterols play a crucial regulatory and structural role in the lateral organization of eukaryotic cell membranes. Cholesterol has been connected to the possible formation of ordered lipid domains (rafts) in mammalian cell membranes. Lipid rafts are composed of lipids in the liquid-ordered (l(o)) phase and are surrounded with lipids in the liquid-disordered (l(d)) phase. Cholesterol and sphingomyelin are thought to be the principal components of lipid rafts in cell and model membranes. We have used fluorescence microscopy and fluorescence recovery after photobleaching in planar supported lipid bilayers composed of porcine brain phosphatidylcholine (bPC), porcine brain sphingomyelin (bSM), and cholesterol to map the composition-dependence of l(d)/l(o) phase coexistence. Cholesterol decreases the fluidity of bPC bilayers, but disrupts the highly ordered gel phase of bSM, leading to a more fluid membrane. When mixed with bPC/bSM (1:1) or bPC/bSM (2:1), cholesterol induces the formation of l(o) phase domains. The fraction of the membrane in the l(o) phase was found to be directly proportional to the cholesterol concentration in both phospholipid mixtures, which implies that a significant fraction of bPC cosegregates into l(o) phase domains. Images reveal a percolation threshold, i.e., the point where rafts become connected and fluid domains disconnected, when 45-50% of the total membrane is converted to the l(o) phase. This happens between 20 and 25 mol % cholesterol in 1:1 bPC/bSM bilayers and between 25 and 30 mol % cholesterol in 2:1 bPC/bSM bilayers at room temperature, and at approximately 35 mol % cholesterol in 1:1 bPC/bSM bilayers at 37 degrees C. Area fractions of l(o) phase lipids obtained in multilamellar liposomes by a fluorescence resonance energy transfer method confirm and support the results obtained in planar lipid bilayers.
Figures





References
-
- Ahmed, S. N., D. A. Brown, and E. London. 1997. On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered phase in model membranes. Biochemistry. 36:10944–10953. - PubMed
-
- Anderson, R. G. W., and K. Jacobson. 2002. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 296:1821–1825. - PubMed
-
- Baird, B., E. D. Sheets, and D. Holowka. 1999. How does the plasma membrane participate in cellular signaling by receptors for immunoglobulin E? Biophys. Chem. 82:109–119. - PubMed
-
- Baumgart, T., T. S. Hess, and W. W. Webb. 2003. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 425:821–824. - PubMed
-
- Bloch, K. 1991. Cholesterol: evolution of structure and function. In Biochemistry of Lipids, Lipoproteins and Membranes. D. E. Vance and J. E. Vance, editors. Elsevier, Amsterdam, The Netherlands. 363–381.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

