Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy
- PMID: 15111440
- PMCID: PMC1304192
- DOI: 10.1016/S0006-3495(04)74375-1
Dynamic elastic modulus of porcine articular cartilage determined at two different levels of tissue organization by indentation-type atomic force microscopy
Abstract
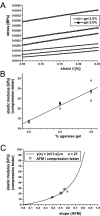
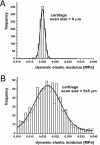
Cartilage stiffness was measured ex vivo at the micrometer and nanometer scales to explore structure-mechanical property relationships at smaller scales than has been done previously. A method was developed to measure the dynamic elastic modulus, |E(*)|, in compression by indentation-type atomic force microscopy (IT AFM). Spherical indenter tips (radius = approximately 2.5 microm) and sharp pyramidal tips (radius = approximately 20 nm) were employed to probe micrometer-scale and nanometer-scale response, respectively. |E(*)| values were obtained at 3 Hz from 1024 unloading response curves recorded at a given location on subsurface cartilage from porcine femoral condyles. With the microsphere tips, the average modulus was approximately 2.6 MPa, in agreement with available millimeter-scale data, whereas with the sharp pyramidal tips, it was typically 100-fold lower. In contrast to cartilage, measurements made on agarose gels, a much more molecularly amorphous biomaterial, resulted in the same average modulus for both indentation tips. From results of AFM imaging of cartilage, the micrometer-scale spherical tips resolved no fine structure except some chondrocytes, whereas the nanometer-scale pyramidal tips resolved individual collagen fibers and their 67-nm axial repeat distance. These results suggest that the spherical AFM tip is large enough to measure the aggregate dynamic elastic modulus of cartilage, whereas the sharp AFM tip depicts the elastic properties of its fine structure. Additional measurements of cartilage stiffness following enzyme action revealed that elastase digestion of the collagen moiety lowered the modulus at the micrometer scale. In contrast, digestion of the proteoglycans moiety by cathepsin D had little effect on |E(*)| at the micrometer scale, but yielded a clear stiffening at the nanometer scale. Thus, cartilage compressive stiffness is different at the nanometer scale compared to the overall structural stiffness measured at the micrometer and larger scales because of the fine nanometer-scale structure, and enzyme-induced structural changes can affect this scale-dependent stiffness differently.
Figures






References
-
- Appleyard, R. C., M. V. Swain, S. Khanna, and G. A. Murrell. 2001. The accuracy and reliability of a novel handheld dynamic indentation probe for analysing articular cartilage. Phys. Med. Biol. 46:541–550. - PubMed
-
- Aspden, R. M., T. Larsson, R. Svensson, and D. Heinegard. 1991. Computer-controlled mechanical testing machine for small samples of biological viscoelastic materials. J. Biomed. Eng. 13:521–525. - PubMed
-
- Bader, D. L., and G. E. Kempson. 1994. The short-term compressive properties of adult human articular cartilage. Biomed. Mater. Eng. 4:245–256. - PubMed
-
- Bueckle, H. 1973. The Science of Hardness Testing and its Research Applications. J.W. Westbrook and H. Conrad, editors. American Society for Metals, Materials Park, Ohio.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

