Mapping the BKCa channel's "Ca2+ bowl": side-chains essential for Ca2+ sensing
- PMID: 15111643
- PMCID: PMC2234491
- DOI: 10.1085/jgp.200409052
Mapping the BKCa channel's "Ca2+ bowl": side-chains essential for Ca2+ sensing
Abstract
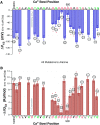
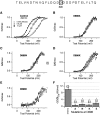
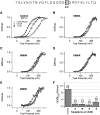
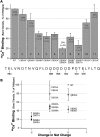
There is controversy over whether Ca(2+) binds to the BK(Ca) channel's intracellular domain or its integral-membrane domain and over whether or not mutations that reduce the channel's Ca(2+) sensitivity act at the point of Ca(2+) coordination. One region in the intracellular domain that has been implicated in Ca(2+) sensing is the "Ca(2+) bowl". This region contains many acidic residues, and large Ca(2+)-bowl mutations eliminate Ca(2+) sensing through what appears to be one type of high-affinity Ca(2+)-binding site. Here, through site-directed mutagenesis we have mapped the residues in the Ca(2+) bowl that are most important for Ca(2+) sensing. We find acidic residues, D898 and D900, to be essential, and we find them essential as well for Ca(2+) binding to a fusion protein that contains a portion of the BK(Ca) channel's intracellular domain. Thus, much of our data supports the conclusion that Ca(2+) binds to the BK(Ca) channel's intracellular domain, and they define the Ca(2+) bowl's essential Ca(2+)-sensing motif. Overall, however, we have found that the relationship between mutations that disrupt Ca(2+) sensing and those that disrupt Ca(2+) binding is not as strong as we had expected, a result that raises the possibility that, when examined by gel-overlay, the Ca(2+) bowl may be in a nonnative conformation.
Figures




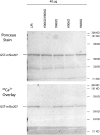
Comment in
-
BK channel news: full coverage on the calcium bowl.J Gen Physiol. 2004 May;123(5):471-3. doi: 10.1085/jgp.200409069. J Gen Physiol. 2004. PMID: 15111642 Free PMC article. No abstract available.
References
-
- Adelman, J.P., K.Z. Shen, M.P. Kavanaugh, R.A. Warren, Y.N. Wu, A. Lagrutta, C.T. Bond, and R.A. North. 1992. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 9:209–216. - PubMed
-
- Atkinson, N.S., G.A. Robertson, and B. Ganetzky. 1991. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 253:551–555. - PubMed
-
- Babu, A., H. Su, and J. Gulati. 1993. The mechanism of Ca2+-coordination in the EF-hand of TnC, by cassette mutagenesis. Adv. Exp. Med. Biol. 332:125–131. - PubMed
-
- Babu, A., H. Su, Y. Ryu, and J. Gulati. 1992. Determination of residue specificity in the EF-hand of troponin C for Ca2+ coordination, by genetic engineering. J. Biol. Chem. 267:15469–15474. - PubMed
-
- Bandyopadhyay, J., J. Lee, J.I. Lee, J.R. Yu, C. Jee, J.H. Cho, S. Jung, M.H. Lee, S. Zannoni, A. Singson, et al. 2002. Calcineurin, a calcium/calmodulin-dependent protein phosphatase, is involved in movement, fertility, egg laying, and growth in Caenorhabditis elegans. Mol. Biol. Cell. 13:3281–3293. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

