Binding of alpha2-macroglobulin to GRAB (Protein G-related alpha2-macroglobulin-binding protein), an important virulence factor of group A streptococci, is mediated by two charged motifs in the DeltaA region
- PMID: 15113281
- PMCID: PMC1133899
- DOI: 10.1042/BJ20030919
Binding of alpha2-macroglobulin to GRAB (Protein G-related alpha2-macroglobulin-binding protein), an important virulence factor of group A streptococci, is mediated by two charged motifs in the DeltaA region
Abstract
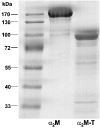
GRAB (Protein G-related alpha2M-binding protein) is a surface protein of group A streptococci and exhibits high affinity for alpha2-macroglobulin (alpha2M), a broad-range protease inhibitor. It is the sole alpha2M-binding protein of group A streptococci that has been shown to promote bacterial virulence in a mouse model of skin infection. The binding site for alpha2M was predicted to be in the N-terminal A domain of GRAB. In the present study, the alpha2M-binding domain was first narrowed down to 34 amino acids (amino acids 34-67) using variable truncated N-terminal GRAB fusion proteins. The sequence of the identified domain was used to design overlapping synthetic peptides of different sizes, which were then immobilized on a membrane and assayed for their alpha2M-binding activity. The peptide screening revealed two binding motifs of ten amino acids length, located in the DeltaA (N-terminal part of the A domain) region (amino acids 34-67) with the sequences PRIIPNGGTL (amino acids 41-50) and NAPEKLALRN (amino acids 56-65) respectively. These motifs were used for systematic mutational analysis by generating synthetic peptides containing individual amino acid substitutions at every position of the mapped binding regions. The results indicated a critical role for the arginine residue at position 42 in the first binding domain and at position 64 in the second binding region. Validation of arginine residues as the critical amino acids for alpha2M binding was achieved by site-directed mutagenesis and binding assays. Competitive inhibition assays with GRAB containing amino acid substitutions R42G (Arg42-->Gly), R64G and R42G/R64G indicated differential contribution of the arginine residues at positions 42 and 64 to alpha2M-binding activity and, thus, their involvement in GRAB-induced virulence.
Figures


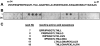




Similar articles
-
Protein GRAB of streptococcus pyogenes regulates proteolysis at the bacterial surface by binding alpha2-macroglobulin.J Biol Chem. 1999 May 28;274(22):15336-44. doi: 10.1074/jbc.274.22.15336. J Biol Chem. 1999. PMID: 10336419
-
A 16-amino acid peptide from human alpha2-macroglobulin binds transforming growth factor-beta and platelet-derived growth factor-BB.Protein Sci. 2000 Oct;9(10):1986-92. doi: 10.1110/ps.9.10.1986. Protein Sci. 2000. PMID: 11106172 Free PMC article.
-
Contribution of protein G-related alpha2-macroglobulin-binding protein to bacterial virulence in a mouse skin model of group A streptococcal infection.J Infect Dis. 2003 Jun 1;187(11):1694-703. doi: 10.1086/375029. Epub 2003 May 15. J Infect Dis. 2003. PMID: 12751026
-
Selective mutations in cloned and expressed alpha-macroglobulin receptor binding fragment alter binding to either the alpha2-macroglobulin signaling receptor or the low density lipoprotein receptor-related protein/alpha2-macroglobulin receptor.J Biol Chem. 1996 Jun 14;271(24):14105-11. doi: 10.1074/jbc.271.24.14105. J Biol Chem. 1996. PMID: 8662881
-
Characterization of the interaction between alpha2-macroglobulin and fibroblast growth factor-2: the role of hydrophobic interactions.Biochem J. 2003 Aug 15;374(Pt 1):123-9. doi: 10.1042/BJ20021655. Biochem J. 2003. PMID: 12755687 Free PMC article.
Cited by
-
alpha-Macroglobulins are present in some gram-negative bacteria: characterization of the alpha2-macroglobulin from Escherichia coli.J Biol Chem. 2008 Oct 17;283(42):28747-56. doi: 10.1074/jbc.M803127200. Epub 2008 Aug 12. J Biol Chem. 2008. PMID: 18697741 Free PMC article.
-
Getting to grips with strangles: an effective multi-component recombinant vaccine for the protection of horses from Streptococcus equi infection.PLoS Pathog. 2009 Sep;5(9):e1000584. doi: 10.1371/journal.ppat.1000584. Epub 2009 Sep 18. PLoS Pathog. 2009. PMID: 19763180 Free PMC article.
-
RivR is a negative regulator of virulence factor expression in group A Streptococcus.Infect Immun. 2013 Jan;81(1):364-72. doi: 10.1128/IAI.00703-12. Epub 2012 Nov 12. Infect Immun. 2013. PMID: 23147037 Free PMC article.
-
Closed Genome Sequence of Noninvasive Streptococcus pyogenes M/emm3 Strain STAB902.Genome Announc. 2014 Aug 28;2(4):e00792-14. doi: 10.1128/genomeA.00792-14. Genome Announc. 2014. PMID: 25169855 Free PMC article.
-
The Streptococcus pyogenes fibronectin/tenascin-binding protein PrtF.2 contributes to virulence in an influenza superinfection.Sci Rep. 2018 Aug 14;8(1):12126. doi: 10.1038/s41598-018-29714-x. Sci Rep. 2018. PMID: 30108238 Free PMC article.
References
-
- Bisno A. L., Stevens D. L. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 1996;334:240–245. - PubMed
-
- Low D. E., Schwartz B., McGeer A. Emerging Pathogens. Proceedings of the 1996 International Congress on Antimicrobial Agents and Chemotherapy. Washington, DC: ASM Press; 1998. The re-emergence of severe group A streptococcal disease: an evolutionary perspective; pp. 93–112.
-
- Davies H. D., McGeer A., Schwartz B., Green K., Cann D., Simor A. E., Low D. E. and The Ontario Group A Streptococcal Study Group. Invasive group A streptococcal infections in Ontario, Canada. N. Engl. J. Med. 1996;335:547–554. - PubMed
-
- Johnson D. R., Wotton J. T., Shet A., Kaplan E. L. A comparison of group A streptococci from invasive and uncomplicated infections: are virulent clones responsible for serious streptococcal infections? J. Infect. Dis. 2002;185:1586–1595. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

