Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models
- PMID: 15113880
- PMCID: PMC400350
- DOI: 10.1128/jvi.78.10.4999-5006.2004
Treatment of transmissible spongiform encephalopathy by intraventricular drug infusion in animal models
Abstract
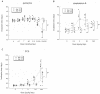
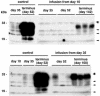
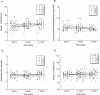
The therapeutic efficacy of direct drug infusion into the brain, the target organ of transmissible spongiform encephalopathies, was assessed in transgenic mice intracerebrally infected with 263K scrapie agent. Pentosan polysulfate (PPS) gave the most dramatic prolongation of the incubation period, and amphotericin B had intermediate effects, but antimalarial drugs such as quinacrine gave no significant prolongation. Treatment with the highest dose of PPS at an early or late stage of the infection prolonged the incubation time by 2.4 or 1.7 times that of the control mice, respectively. PPS infusion decreased not only abnormal prion protein deposition but also neurodegenerative changes and infectivity. These alterations were observed within the brain hemisphere fitted with an intraventricular infusion cannula but not within the contralateral hemisphere, even at the terminal disease stage long after the infusion had ended. Therapeutic effects of PPS were also demonstrated in mice infected with either RML agent or Fukuoka-1 agent. However, at doses higher than that providing the maximal effects, intraventricular PPS infusion caused adverse effects such as hematoma formation in the experimental animals. These findings indicate that intraventricular PPS infusion might be useful for the treatment of transmissible spongiform encephalopathies in humans, providing that the therapeutic dosage is carefully evaluated.
Figures
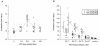


Similar articles
-
Experimental treatments for human transmissible spongiform encephalopathies: is there a role for pentosan polysulfate?Expert Opin Biol Ther. 2007 May;7(5):713-26. doi: 10.1517/14712598.7.5.713. Expert Opin Biol Ther. 2007. PMID: 17477808 Review.
-
Protease-resistant PrP and PrP oligomers in the brain in human prion diseases after intraventricular pentosan polysulfate infusion.Neuropathology. 2012 Apr;32(2):124-32. doi: 10.1111/j.1440-1789.2011.01245.x. Epub 2011 Aug 1. Neuropathology. 2012. PMID: 21801238
-
Continuous intraventricular infusion of pentosan polysulfate: clinical trial against prion diseases.Neuropathology. 2009 Oct;29(5):632-6. doi: 10.1111/j.1440-1789.2009.01058.x. Neuropathology. 2009. PMID: 19788637 Clinical Trial.
-
Comparison of CR36, a new heparan mimetic, and pentosan polysulfate in the treatment of prion diseases.J Gen Virol. 2007 Mar;88(Pt 3):1062-1067. doi: 10.1099/vir.0.82286-0. J Gen Virol. 2007. PMID: 17325382
-
Pentosan polysulfate as a prophylactic and therapeutic agent against prion disease.IDrugs. 2003 May;6(5):470-8. IDrugs. 2003. PMID: 12789602 Review.
Cited by
-
Prion diseases: immunotargets and therapy.Immunotargets Ther. 2016 Jun 16;5:57-68. doi: 10.2147/ITT.S64795. eCollection 2016. Immunotargets Ther. 2016. PMID: 27529062 Free PMC article. Review.
-
In vivo detection of prion amyloid plaques using [(11)C]BF-227 PET.Eur J Nucl Med Mol Imaging. 2010 May;37(5):934-41. doi: 10.1007/s00259-009-1314-7. Epub 2009 Dec 17. Eur J Nucl Med Mol Imaging. 2010. PMID: 20016895 Clinical Trial.
-
Pentosan polysulfate induces low-level persistent prion infection keeping measurable seeding activity without PrP-res detection in Fukuoka-1 infected cell cultures.Sci Rep. 2022 May 13;12(1):7923. doi: 10.1038/s41598-022-12049-z. Sci Rep. 2022. PMID: 35562591 Free PMC article.
-
Identification of chemoattractive factors involved in the migration of bone marrow-derived mesenchymal stem cells to brain lesions caused by prions.J Virol. 2011 Nov;85(21):11069-78. doi: 10.1128/JVI.05318-11. Epub 2011 Aug 3. J Virol. 2011. PMID: 21813601 Free PMC article.
-
Amphotericin B induces glial cell line-derived neurotrophic factor in the rat brain.J Vet Med Sci. 2014 Oct;76(10):1353-8. doi: 10.1292/jvms.14-0160. Epub 2014 Oct 3. J Vet Med Sci. 2014. PMID: 25283947 Free PMC article.
References
-
- Brown, P. 2002. Drug therapy in human and experimental transmissible spongiform encephalopathy. Neurology 58:1720-1725. - PubMed
-
- Brown, P., M. Preece, J. P. Brandel, T. Sato, L. McShane, I. Zerr, A. Fletcher, R. G. Will, M. Pocchiari, N. R. Cashman, J. H. d'Aignaux, L. Cervenakova, J. Fradkin, L. B. Schonberger, and S. J. Collins. 2000. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 55:1075-1081. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

