Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase
- PMID: 15113886
- PMCID: PMC400327
- DOI: 10.1128/jvi.78.10.5045-5055.2004
Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase
Abstract
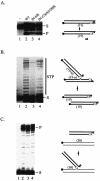
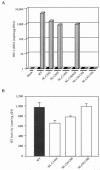
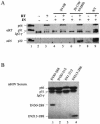
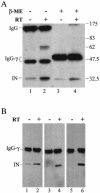
Retroviral integrase catalyzes the essential step of integrating a double-stranded DNA copy of the viral genome into a host cell chromosome. Mutational studies have revealed that integrase is involved in additional steps of viral replication, but the mechanism for the pleiotropic effect is not well characterized. Since Cys residues generally play crucial roles in protein structure and function, we introduced Cys-to-Ser substitutions at positions 56, 65, and 130 of human immunodeficiency virus type 1 (HIV-1) integrase to determine their effects on integration activity and viral replication. None of the substitutions significantly affected the enzymatic activities in vitro. When introduced into the NL4-3 molecular clone of HIV-1, mutant viruses encoding Cys mutations at positions 56 and 65 of integrase replicated similarly to the wild-type virus in CD4(+)-T-cell lines, whereas the C130S-containing virus was noninfectious. The entry and postintegration steps of the viral life cycle for all mutant viruses were normal, and all had particle-associated reverse transcriptase (RT) activity. However, early reverse-transcribed DNA products were absent in the lysate of cells infected with the C130S mutant virus, indicating that the mutation abolished the ability of the virus to initiate endogenous reverse transcription. Coimmunoprecipitation using purified integrase and RT showed that the C-terminal domain of wild-type HIV-1 integrase interacted with RT. The interaction between integrase and RT was not affected in the presence of a reducing or alkylating agent, suggesting that the interaction did not involve a disulfide linkage. The C130S substitution within the core region may disrupt the protein recognition interface of the C-terminal domain and abolish its ability to interact with RT. Our results indicate that integrase plays an important role during the reverse-transcription step of the viral life cycle, possibly through physical interactions with RT.
Figures



Similar articles
-
Interaction between Reverse Transcriptase and Integrase Is Required for Reverse Transcription during HIV-1 Replication.J Virol. 2015 Dec;89(23):12058-69. doi: 10.1128/JVI.01471-15. Epub 2015 Sep 23. J Virol. 2015. PMID: 26401032 Free PMC article.
-
Contribution of the C-terminal tri-lysine regions of human immunodeficiency virus type 1 integrase for efficient reverse transcription and viral DNA nuclear import.Retrovirology. 2005 Oct 18;2:62. doi: 10.1186/1742-4690-2-62. Retrovirology. 2005. PMID: 16232319 Free PMC article.
-
Critical Contribution of Tyr15 in the HIV-1 Integrase (IN) in Facilitating IN Assembly and Nonenzymatic Function through the IN Precursor Form with Reverse Transcriptase.J Virol. 2016 Dec 16;91(1):e02003-16. doi: 10.1128/JVI.02003-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795445 Free PMC article.
-
[Viral and host factors affecting efficient revere transcription of HIV-1 genome].Uirusu. 2011 Jun;61(1):73-80. doi: 10.2222/jsv.61.73. Uirusu. 2011. PMID: 21972558 Review. Japanese.
-
Molecular mechanisms of HIV integration and therapeutic intervention.Expert Rev Mol Med. 2007 Feb 26;9(6):1-19. doi: 10.1017/S1462399407000257. Expert Rev Mol Med. 2007. PMID: 17320002 Review.
Cited by
-
NKNK: a New Essential Motif in the C-Terminal Domain of HIV-1 Group M Integrases.J Virol. 2020 Sep 29;94(20):e01035-20. doi: 10.1128/JVI.01035-20. Print 2020 Sep 29. J Virol. 2020. PMID: 32727879 Free PMC article.
-
Natural single-nucleotide polymorphisms in the 3' region of the HIV-1 pol gene modulate viral replication ability.J Virol. 2014 Apr;88(8):4145-60. doi: 10.1128/JVI.01859-13. Epub 2014 Jan 29. J Virol. 2014. PMID: 24478432 Free PMC article.
-
Retroviral Integrase: Then and Now.Annu Rev Virol. 2015 Nov;2(1):241-64. doi: 10.1146/annurev-virology-100114-055043. Annu Rev Virol. 2015. PMID: 26958915 Free PMC article. Review.
-
The N-end rule and retroviral infection: no effect on integrase.Virol J. 2013 Jul 13;10:233. doi: 10.1186/1743-422X-10-233. Virol J. 2013. PMID: 23849394 Free PMC article.
-
HIV-1 integrase is capable of targeting DNA to the nucleus via an importin alpha/beta-dependent mechanism.Biochem J. 2006 Sep 15;398(3):475-84. doi: 10.1042/BJ20060466. Biochem J. 2006. PMID: 16716146 Free PMC article.
References
-
- Aldovini, A., and B. D. Walker. 1990. Techniques in HIV research. Stockton Press, New York, N.Y.
-
- Ausubel, F. A., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1999. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

