Interaction between human immunodeficiency virus type 1 reverse transcriptase and integrase proteins
- PMID: 15113887
- PMCID: PMC400328
- DOI: 10.1128/jvi.78.10.5056-5067.2004
Interaction between human immunodeficiency virus type 1 reverse transcriptase and integrase proteins
Abstract
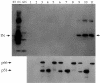
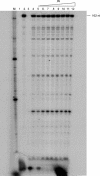
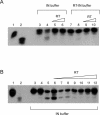
Reverse transcriptase (RT) and integrase (IN) are two key catalytic enzymes encoded by all retroviruses. It has been shown that a specific interaction occurs between the human immunodeficiency virus type 1 (HIV-1) RT and IN proteins (X. Wu, H. Liu, H. Xiao, J. A. Conway, E. Hehl, G. V. Kalpana, V. R. Prasad, and J. C. Kappes, J. Virol. 73:2126-2135, 1999). We have now further examined this interaction to map the binding domains and to determine the effects of interaction on enzyme function. Using recombinant purified proteins, we have found that both a HIV-1 RT heterodimer (p66/p51) and its individual subunits, p51 and p66, are able to bind to HIV-1 IN. An oligomerization-defective mutant of IN, V260E, retained the ability to bind to RT, showing that IN oligomerization may not be required for interaction. Furthermore, we report that the C-terminal domain of IN, but not the N-terminal zinc-binding domain or the catalytic core domain, was able to bind to heterodimeric RT. Deletion analysis to map the IN-binding domain on RT revealed two separate IN-interacting domains: the fingers-palm domain and the carboxy-terminal half of the connection subdomain. The carboxy-terminal domain of IN alone retained its interaction with both the fingers-palm and the connection-RNase H fragments of RT, but not with the half connection-RNase H fragment. This interaction was not bridged by nucleic acids, as shown by micrococcal nuclease treatment of the proteins prior to the binding reaction. The influences of IN and RT on each other's activities were investigated by performing RT processivity and IN-mediated 3' processing and joining reactions in the presence of both proteins. Our results suggest that, while IN had no influence on RT processivity, RT stimulated the IN-mediated strand transfer reaction in a dose-dependent manner up to 155-fold. Thus, a functional interaction between these two viral enzymes may occur during viral replication.
Figures







Similar articles
-
Intramolecular chimeras of the p51 subunit between HIV-1 and FIV reverse transcriptases suggest a stabilizing function for the p66 subunit in the heterodimeric enzyme.Biochemistry. 1999 Feb 2;38(5):1633-42. doi: 10.1021/bi9821162. Biochemistry. 1999. PMID: 9931031
-
Requirement for integrase during reverse transcription of human immunodeficiency virus type 1 and the effect of cysteine mutations of integrase on its interactions with reverse transcriptase.J Virol. 2004 May;78(10):5045-55. doi: 10.1128/jvi.78.10.5045-5055.2004. J Virol. 2004. PMID: 15113886 Free PMC article.
-
The nature of the N-terminal amino acid residue of HIV-1 RNase H is critical for the stability of reverse transcriptase in viral particles.J Virol. 2015 Jan 15;89(2):1286-97. doi: 10.1128/JVI.02312-14. Epub 2014 Nov 12. J Virol. 2015. PMID: 25392207 Free PMC article.
-
Human Immunodeficiency Virus type 1 reverse transcriptase.Biol Chem Hoppe Seyler. 1996 Feb;377(2):97-120. Biol Chem Hoppe Seyler. 1996. PMID: 8868066 Review.
-
Buried surface analysis of HIV-1 reverse transcriptase p66/p51 heterodimer and its interaction with dsDNA template/primer.J Mol Recognit. 1994 Jun;7(2):157-61. doi: 10.1002/jmr.300070212. J Mol Recognit. 1994. PMID: 7530020 Review.
Cited by
-
Importin alpha3 interacts with HIV-1 integrase and contributes to HIV-1 nuclear import and replication.J Virol. 2010 Sep;84(17):8650-63. doi: 10.1128/JVI.00508-10. Epub 2010 Jun 16. J Virol. 2010. PMID: 20554775 Free PMC article.
-
Targeting human immunodeficiency virus type 1 assembly, maturation and budding.Drug Target Insights. 2007;2:159-82. Epub 2007 Jul 20. Drug Target Insights. 2007. PMID: 21901072 Free PMC article.
-
Convergent evolution of integration site selection upstream of tRNA genes by yeast and amoeba retrotransposons.Nucleic Acids Res. 2018 Aug 21;46(14):7250-7260. doi: 10.1093/nar/gky582. Nucleic Acids Res. 2018. PMID: 29945249 Free PMC article.
-
Human immunodeficiency virus type 1 employs the cellular dynein light chain 1 protein for reverse transcription through interaction with its integrase protein.J Virol. 2015 Apr;89(7):3497-511. doi: 10.1128/JVI.03347-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568209 Free PMC article.
-
Correlation of recombinant integrase activity and functional preintegration complex formation during acute infection by replication-defective integrase mutant human immunodeficiency virus.J Virol. 2012 Apr;86(7):3861-79. doi: 10.1128/JVI.06386-11. Epub 2012 Jan 25. J Virol. 2012. PMID: 22278243 Free PMC article.
References
-
- Andrake, M. D., and A. M. Skalka. 1995. Multimerization determinants reside in both catalytic core and C terminus of avian sarcoma virus integrase. J. Biol. Chem. 270:29299-29306. - PubMed
-
- Arts, E. J., X. Li, Z. Gu, L. Kleiman, M. A. Parniak, and M. A. Wainberg. 1994. Comparison of deoxyoligonucleotide and tRNA(Lys-3) as primers in an endogenous human immunodeficiency virus-1 in vitro reverse transcription/template-switching reaction. J. Biol. Chem. 269:14672-14680. - PubMed
-
- Asante-Appiah, E., and A. M. Skalka. 1999. HIV-1 integrase: structural organization, conformational changes, and catalysis. Adv. Virus Res. 52:351-369. - PubMed
-
- Beard, W. A., and S. H. Wilson. 1993. Kinetic analysis of template-primer interactions with recombinant forms of HIV-1 reverse transcriptase. Biochemistry 32:9745-9753. - PubMed
-
- Bukrinsky, M. I., N. Sharova, T. L. McDonald, T. Pushkarskaya, W. G. Tarpley, and M. Stevenson. 1993. Association of integrase, matrix, and reverse transcriptase antigens of human immunodeficiency virus type 1 with viral nucleic acids following acute infection. Proc. Natl. Acad. Sci. USA 90:6125-6129. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

